Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sara Zafar1, Asma Ashraf2, M. Yasin Ashraf*3, Farkhanda Asad2, Shagufta Perveen1, M. Amir Zafar1 and Andleeb Shahzadi4
Received: September 29, 2018; Published: October 04, 2018
*Corresponding author: M Yasin Ashraf, NIAB, Faisalabad, Pakistan
DOI: 10.26717/BJSTR.2018.09.001829
Purpose: Present study was conducted to prepare simple, low cost and quick in action silver nanoparticles (Ag NPs) using silver nitrate and eco-friendly biological material (leaves of Ficus benjamina), for four pathogenic bacteria.
Methods: The leaf extract of Ficus benjamina was prepared by soaking 10 g washed leaves in 90 mL of distilled water and were boiled at 90 °C for 20 minutes; filtrate was stored at 4°C. Silver nanoparticles were prepared by adding 10 mL of filtrate in 50 mL of 1 mM silver nitrate solution, sonicated at 60°C for 60 minutes. A change in the color from yellow to brown indicated the formation of Ag NPs. Ag NPs were characterized by SEM, XRD and FTIR techniques and their antibacterial activity was estimated by disc diffusion method.
Results: The solution changed from a light yellowish to dark brown, caused by a reduction in the number of silver ions, confirming the synthesis of Ag NPs. The XRD spectrum revealed the crystalline Ag NPs were of 13.2 nm size. The FTIR and SEM images showed that the Ag NPs were fairly spherical. The disc-diffusion showed that prepared Ag NPs strongly inhibited the pathogenic activities of Bacillus subtilis, Pasteurella multocida, Staphylococcus aureus and Enterobacter aerogenes. The inhibition zone ranged from 7 mm to 13 mm.
Conclusion: Present findings indicated that the green synthesis of Ag NPs has many advantages over other chemical procedures, offering enhanced safety, eco-friendlier and more superior in results.
Abbreviations: AgNPs: silver nanoparticles; XRD: X-Ray diffraction; SEM: scanning electron microscope; FTIR: Fourier Transform Infrared Spectroscopy
Nanotechnology is an emerging field science having vast scope in research and development with the aim of production and implications of new materials having nanoscale dimension [1, 2]. Nanoparticles are atoms with size up to 100 nm [3] showing fascinating properties established on particular features such as size, utility and morphology. Characterization is performed to access different properties of the nanoparticles [4, 5]. Literature suggested a variety of nanoparticles utilization for different purposes like treatment of diseases and disorders but silver nano particles showed dominant antimicrobial activity [1,6]. They also have anti-inflammatory and anti-viral activity [7]. Silver is also used in silver-based ointments to cure wounds, burns and medical related strategies that are associated with polymers saturated with silver [8]. Utilization of silver (Ag) nanoparticles, particularly prepared with medicinal plants is a novel approach to treat microbial pathogen, furthermore, secondary metabolites in plant extracts play an important role in the synthesis of nano silver particles having strong antibacterial potential [9].
A large number of different protocols including chemical, physical and biological are used for the synthesis of nanoparticles [10, 11]. By using different types of chemical procedures large number of nanoparticles can be prepared in short time, this method requires finishing agents for the stabilization of the size of the particles but non-toxic and environmentally friendly protocol is necessary for the formation of nanoparticles and to get rid of fatal chemicals as derivatives [12]. Plant extracts contain reducing and antioxidant properties which cause the reduction of metal salts to their respective nanoparticles [13]. Therefore, demand of green nanotechnology is increased [14-16]. In the current year, formation of Ag nanoparticles by applying green chemistry with natural reducing, capping and stabilizing agents become a major attention for researcher [17]. Green chemistry production method of nanoparticles is less costly and ecological save for large scale production [18]. This study explains the production of Ag NPs through bio-reduction method using Ficus benjamina leaf extract. Different types of phytochemical have been involved in reducing of silver ions in silver nitrate solution which lead to the formation of Ag NPs. Furthermore, synthesized silver- nanoparticles were checked with different analytical approaches and their antibacterial action was checked through the disc diffusion method.
The leaves of Ficus benjamina were washed with distilled water three times. Dried homogeneous 10 g leaves censored into many sections were soaked in 90 mL of distilled water. The leaves were kept boiling at 90 °C for 20 minutes, extract was collected and stored at 4 °C.
Silver nanoparticles were prepared by adding 10 mL of filtrate in 50 ml of 1 mM silver nitrate solution [19]. Sonicate the solution at about 60 °C for 60 minutes. A change in the color from yellow (bright) to brown (darkish) indicated the formation of silver nanoparticles.
The dimeter of crystalline silver nanoparticles (Ag NPs) was calculated by using the X-Ray diffraction by Scherrer's equation
t = k λ / B cosθ,
where, K = (0.92), t = crystal size, A = (1.5418A°).
The SEM (scanning electron microscope) was used for scanning the surface of sample. The chemical nature of silver synthesized nanoparticles to classify the biomolecules responsible for reduction was developed by Fourier Transform Infrared Spectroscopy (FTIR) technique using FTIR RX1-Perkin Elmer in the wavelength range 4000-400 cm-1.
The antibacterial activity of prepared silver nanoparticles (Ag NPs) was examined using Kirby-Bauer standard disc diffusion method [20]. The application of bacterial suspension (100 θl) on the surface of a nutrient agar plate was done overnight. Antibacterial disc (Gentamicin) and different concentrations of Ag NPs 50, 100, 150, 200 θl/disc were placed on the agar plate. After incubation at 37 °C for 24h, the inhibition zones were noticed around each disc.
The XRD diffraction spectrum of the "green" Ag NPs expressed peaks at 20 (32,595, 46, 555, 54,815, 57,605, 66,575) equivalent to Ag (101), (111), (200), (202) and (220) planes of standard XRD. The The XRD design clearly explained that prepared Ag NPs possess a cubic crystalline structure [21]. The XRD parameters revealed a strong plane of Ag (101) and other weaker planes (Figure 1). The average size (13.2 nm) of the crystalline Ag NPs was confirmed by X-ray diffraction.
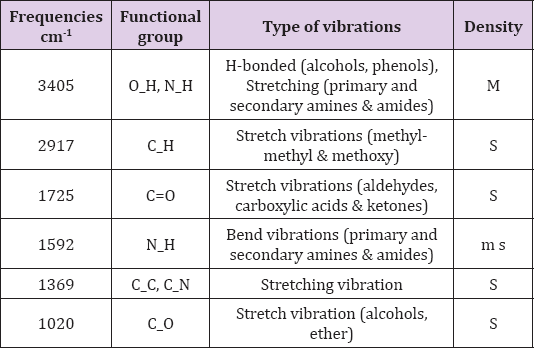
FTIR spectroscopy is an effective strategy for determining the major functional groups (reduced bio-molecules) necessary for the "green" synthesis of NPs via the reduction and stabilization of Ag+ ions (Figure 2). The FTIR spectrum revealed a number of absorption bands [22]. At 3405 cm-1 absorption band confirmed the presence of O-H vibration of phenol, alcohol and phenol with stretching linkage of N-H of primary, secondary amides and amines (Table 1) . Similarly, a peak at 2917 cm-1 was confirmed, representing stretching vibrations of methyl groups (C-H) [23]. The stretching sensations of peak at 1725 cm-1 showed the presence of (C=O) functional groups of carboxylic acids in addition with aldehydes and ketones. The carbonyl stretching absorption was found to be one of the strongest IR absorptions, and is a valuable component in terms of structure. A peak of Ag NPs was observed at band of 1592 cm-1, showing the occurance of N-H primary and secondary amides with amines, in addition to bending vibrations (Figure 2). A peak at 1369 cm-1 reflected bands of stretching of C-C and C-N groups, mostly present in proteins. Presence of alcohol and ether groups with C-O streching vibrations was observed at the absorbing peak of 1020 cm-1 [24]. These biomolecules are responsible in the reduction (Ag ions) and stabilizing of the Ag NPs.
Table 1: FTIR result showed stretching and bending vibration of compounds with functional group. Frequencies
SEM analysis verified the presence of Ag NPs created by treating silver nitrate solution with Ficus benjamina leaf extract. The results revealed that the Ag NPs were mostly of uniform size 70 nm and fairly spherical. The shape and size of NPs are extremely dependent on the concentration of the leaf extract and the ratio of silver nitrate (AgNO3) to Ficus benjamina extract (Figure 3).
The antibacterial activity of the synthesized Ag NPs was determined by disc-diffusion method. The nanoparticles expressed various level of antibacterial activity against two bacterial strains of Gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus) and two strains of Gram-negative bacteria (Pasteurella multocida, Enterobacter aerogene,), depending on the diameter of the activity zone (Figure 4) and (Table 2). The antibacterial activity of Ag NPs differs by changing the concentration of the stock solution from 50 θl to 200 θl. With a solution of 200 θl Ag NPs the highest growth inhibition effect of 13 mm was observed against Bacillus subtilis, Enterobacter aerogenes, Staphylococcus aureus and Pasteurella multocida (Figure 4) and (Table 2). The silver nanoparticles adhere to the negatively charged cell surface, and impair membrane permeability, osmoregulation, respiration and electron transport. The Ag+ ions in silver nanoparticles lead directly to the denaturation of proteins in bacteria by bonding with sulfhydryl groups. Silver ions penetrate the cell wall and cause severe damage to the bacteria.
Ficus benjamina leaf extract was used for the preparation of Ag NPs because the bio-molecules in the leaf extract are effective in stabilizing the Ag NPs [21]. A change in color of the extract with AgNO3 solution indicated the reduction in silver ions that was due to the excitation of surface plasmon vibrations which confirmed the formation of Ag NPs [22]. Characterization of nanoparticles is necessary to know whether we prepared the desired nanoparticles for particular applications. By using XRD analysis formation of desired silver particles was determined and spectral analysis in different planes showed the nature of crystallization of particles and variations in their size and shape (Figure 1). Each Ag NP prepared with 13.2 nm average size exhibiting a crystalline structure in cubic form corresponding to 20 values [21]. Biological molecules involved in stabilizing the Ag NPs were identified by FTIR dimension (Figure 2). The prominent peaks corresponded to the amines, alcohol, amide, phenol methyl and ether linkages (Figure 2) . Other peaks suggested the existence of ketones, carboxylic acids and aldehydes [23]. The bending and stretching sensations revealed that bio-molecules like alcoholic groups, polyphenols, proteins and carboxylic acids are bound together by Ag NPs [24]. These nano molecules are inter connected with the reduction and controlling of AgNO3 and of the Ag particles.
SEM image analysis of surface morphology of nanoparticles revealed moderately spherical form with a diameter of 70 nm (Figure 3) as suggested in the literature [25]. Ficus benjamina leaf extract -mediated Ag NPs exhibited strong antibacterial activity. In this study, bacterial Gram-positive and Gram-negative strains were used to ascertain the inhibitory potential of Ag NPs and results showed that they had strong antibacterial activity and prepared Ag NPs varies according to the sizes of bacteria. Antibacterial activity increases with decreasing size of Ag NPs [26]. The findings of present study showed that the highest zone of inhibition of 13 mm with 200 θl Ag NPs solution against Pasteurella multocida, Enterobacter aerogenes, Staphylococcus aureus and Bacillus subtilis (Figure 4) (Table 2). Ag NPs are capable of adhering and entering the the cell wall, causing changes in the membrane permeability leading to cell death [27]. Silver ions are absorbed by bacterial cells which inhibited normal cell functions and caused cell damage. The overall finding of presnt study illustrated that Ficus benjamina Ag NPs exhibited strong antibacterial activity against selected strains of pathogenic bacteria.
Present findings suggested that the green synthesis of Ag NPs has many advantages over other chemical procedures, offering enhanced safety, eco-friendlier and more superior in results. The characterization of Ag NPs was done through XRD spectra, showed that the Ag NPs are strongly dependent on the concentration of Ficus benjamina leaf extract and relative amount of silver nitrate to leaf extract. SEM descriptions revealed the Ag NPs were spherical in shape and FTIR analysis suggested that bio-molecules are involved in the silver ion reduction and in stabilizing Ag NPs. Green synthesized Ag NPs possess greater antibacterial activity against infectious bacteria illustrating an innovative way to a wide array of applications.


