Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yang Gu*
Received: June 26, 2018; Published: July 03, 2018
*Corresponding author: Yang Gu, Assistant Professor and Oral Pathologist, Director of Oral Pathology Clinic, Department of Oral and Maxillofacial Sciences, Faculty of Dentistry, Dalhousie University, Canada
DOI: 10.26717/BJSTR.2018.06.001332
Aims: The paper reorganized new findings in the field of osteoblastogenesis and osteoclastogenesis for clinicians to identify systemic and local risks of oral and maxillofacial osteogenic conditions (OMOCs) that are not related to genetic diseases.
Method: The non-systemic review was undertaken by searching pertinent key wards in English literature. Case report and publications with weak levels of evidence were excluded.
Results: Coupling factors from endocrine and paracrine systems affect osteoblastogenesis and osteoclastogenesis in different pathways. Levels of hormones change with age or by medical conditions. Cytokine accumulations are triggered by the local mechanical force or ischemia. Osteoblasts take a leading role in the maturation of osteoclasts, while the activity of osteoclasts could be an initial event in a local osteogenic lesion. The entangling relation between osteoblasts and osteoclasts derives some patterns for OMOCs radiographically.
Discussion: A triple-hit frame composited by timings, coupling factors from endocrine and paracrine systems is an approachable method to explore local and systemic risks for OMOCs.
Keywords: Osteoblast; Osteoclast; Hormone; Cytokine; Radiolucency; Radiopacity
Abbrevations: OPG: Osteoprotegerin; RANKL: Nuclear Factor-kB Ligand; GI: Gastrointestinal System; S1P: Sphingosine 1-Phosphate; TNF: Tumor Necrosis Factor; IL: Interleukin; FasL: Fas Ligand; PDGF: Platelet-Derived Growth Factor; VEGF: Vascular Endothelial Growth Factor
The ratio of nuclear factor kB ligand (RANKL) to osteoprotegerin (OPG) is not the only tool to explore the interaction between osteoblasts and osteoclasts. Sphingosine 1-phosphate (S1P) and Sclerostin work on osteoblasts in the opposite way. Eight hormones (Growth Hormone, Calcitriol, Androgen, Estrogen, Calcitonin, Thyroxin, Cortisol and Parathyroid Hormone) regulate behaviors of osteoblasts and osteoclasts at different levels. Seven cytokines (Macrophage colony-stimulating factor, Tumor necrosis factoralpha, Histamine, Interleukin-1, 4, 12 and 13) affect their behaviors in different pathways. Lifespans of osteoblasts, osteoclasts, fibroblasts and endothelium are different, while hormone levels of Growth Hormone, Estrogen, Androgen and Thyroxin change with aging. The unbalanced behavior of osteoblasts and osteoclasts could induce the excessive bone formation and the abnormal bone resorption, which project on a radiography as radiolucency or radiopacity or mixed lucent-opaque lesions. The paper tried to reorganize new findings for clinicians to identify systemic and local risks of non-genetic oral and maxillofacial osteogenic conditions (OMOCs).
The non-systematic review identified recent original research papers, systematic reviews, meta-analysis articles and narrative reviews from author input supplemented by the PubMed, Google Scholar and PLOS ONE. Keywords included osteoblasts, osteoclasts, hormone, cytokine, radiolucency, radiopacity and oral and maxillofacial osteogenic conditions. Case reports and publications with weak levels of evidence were excluded.
Osteoblasts differentiate from mesenchymal osteoprogenitors who are governed by the RUNX2/CBFA1 transcription factor network and the WNT/ beta-catenin signal pathway [1]. Osteoblasts synthesize, transport and arrange proteins of bone matrix and initiate the process of mineralization. The osteoblast will transform into an osteocyte when it is surrounded by newly deposited bone matrix. Common markers for active osteoblasts are Alkaline Phosphatase (ALP) and Osteocalcin [2,3]. Osteoclast precursors differentiate from hematopoietic progenitor cells that are probably governed by the ERK-Akt pathway through c-Fms and c-Kit receptors [4]. An osteoclast precursor (mononuclear osteoclast) will transform into a mature osteoclast (multinuclear osteoclast) when the NF-kB signaling and the tyrosine kinase receptor are activated. Multinuclear osteoclasts digest proteins of bone matrix and initiate the process of demineralization. Common markers for mature osteoclasts are Tartrate-Resistant Acid Phosphatase (TRAP) and Cathepsin K [2,5].
An osteoblast presents a receptor activator of nuclear factor-kB ligand (RANKL) and Macrophage colony-stimulating factor (M-CSF) on the surface, while a mononuclear osteoclast expresses RANKL receptor (RANK) and M-CSF receptor (a type of tyrosine kinase receptors). When they bind together the NF-kB signaling and the tyrosine kinase receptor are activated. A multinuclear osteoclast matures1. Osteoblasts take a leading role in the maturation of osteoclasts.
Illustration 1: Triple-hit frame: Interactions between osteoblasts and osteoclasts are regulated by coupling factors from endocrine and paracrine systems (in color). GH: growth hormone; IL: interleukin; PTH: parathyroid hormone; TNF: tumor necrosis factor; OPG: osteoprotegerin; RANKL: nuclear factor-kB ligand; RANK: RANKL receptor; LDLR: low-density lipoprotein receptor; M-CSF: macrophage colony- stimulating factor; M-CSFR: M-CSF receptor; S1P: Sphingosine 1-phosphate; S1PR: S1P receptor.
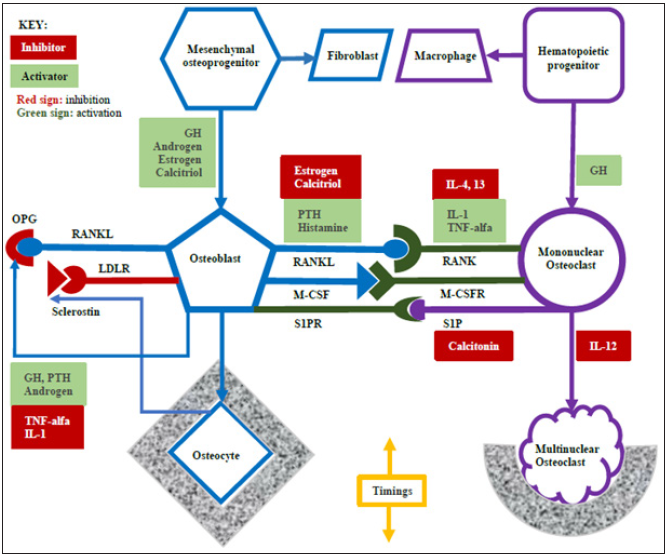
An osteoblast also produces osteoprotegerin (OPG), which is a “decoy” receptor binding RANKL. It blocks RANKL to bind RANK [6]. It is a negative control to the maturation of osteoclasts. An osteoclast produces Sphingosine 1-phosphate (S1P), which binds the S1P receptor of an osteoblast [7]. The signal promotes osteoblasts to produce bone matrix. It is a positive control to the function of osteoblasts. However, an osteocyte produces Sclerostin to bind the low-density lipoprotein receptor (LDLR)-related protein of an osteoblast [8]. It inhibits osteoblasts to produce bone matrix. It is a negative control to the function of osteoblasts.
RANKL and OPG, both from osteoblasts, oppose one another. S1P and Sclerostin work on osteoblasts but in the opposite way. Who controls the direction? They are endocrine molecules (e.g. hormones) and paracrine molecules (e.g. cytokines).
Bone Formation as a Priority:
a) Growth hormone (GH) binds GH receptors (GHR) of osteoblasts and osteoclast precursors to promote their differentiation but also stimulates osteoblasts to produce OPG resulting in the bone formation as a priority [9].
b) Estrogen binds estrogen receptors (ER) of osteoprogenitors to promote the differentiation of osteoblasts and binds ERs of osteoblasts to inhibit osteoblasts to present RANKL resulting in the bone formation as a priority [10,11].
c) Androgen binds an androgen receptor (AR) of osteoprogenitors to promote the differentiation of osteoblasts and binds ERs to stimulate osteoblasts to produce OPG resulting in the bone formation as a priority [12,13].
Mineral Homeostasis as a Priority:
a) Parathyroid hormone (PTH) binds PTH receptors of osteoblasts to increase the expression of RANKL and to decrease the production of OPG (E/C) resulting in the maturation of osteoclasts and following with the bone resorption as a priority, but another function is activating alpha-hydroxylase to promote Calcitriol formation. Hypocalcemia is the stimulator of PTH [14].
b) Calcitriol binds Vitamin D receptors (VDR) of osteoprogenitors to promote the differentiation of osteoblasts [15], also works as a suppressor of inhibiting osteoblasts to express RANKL [16] resulting in the bone formation as a priority, but the well-known function is helping the intestine and kidneys to absorb calcium. Hypocalcemia and PTH are stimulators of Calcitriol.
c) Calcitonin binds a Calcitonin receptor (CTR) of a mature osteoclast to inhibit the production of Sphingosine 1 phosphate (S1P) [7,17]. However, the S1P binds an S1P receptor of an osteoblast to promote the production of bone matrix. Hypercalcemia is the stimulator of Calcitonin.
Negative Effect on Bone:
a) Thyroxin binds thyroid hormone receptors (THR) of osteoblasts and osteoclasts to promote their functions, but it shortens the bone remodeling cycle resulting in a loss of about 10% bone mass per cycle [18].
b) Cortisol inhibits osteoclastogenesis and osteoblastogenesis by the FOXO antioxidant pathway and binds the Glucocorticosteroid receptor (GR) of endothelium to inhibit angiogenesis resulting in the osteoporosis [19,20].
Hormones control the behavior of osteoblasts and osteoclasts through specific receptors in different directions. The natural course of bone formation and bone resorption is changed by the imbalance of hormone levels. Aetiologies of the abnormal secretion of hormones are complicated. For example, hypocalcemia usually is associated with Gastrointestinal conditions and renal dysfunctions, while hypercalcemia usually is related to renal failure and systemic granulomatous diseases. Practically, the systemic risks of OMOCs could be identified from the medical history and the laboratory examination.
Stromal cells (including endothelium and fibroblast) and inflammatory cells produce cytokines when facing ischemia and inflammation.
Table 1: Pathways of hormones and cytokines working on osteoblasts and osteoclasts and alterations caused by common medical conditions and drugs.
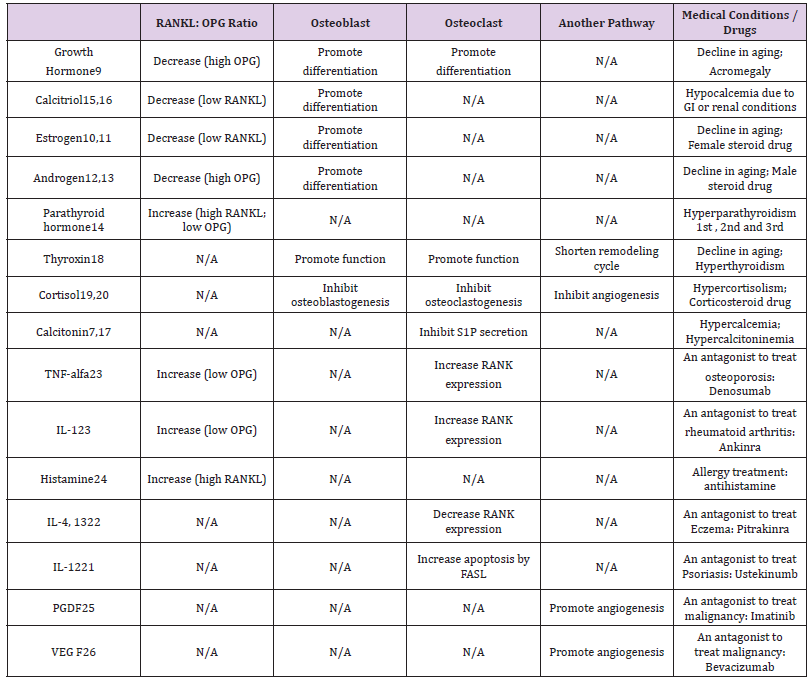
Note: OPG: osteoprotegerin; RANKL: nuclear factor-kB ligand; GI: gastrointestinal system; S1P: Sphingosine 1-phosphate; TNF: tumor necrosis factor; IL: interleukin; FasL: Fas ligand; PDGF: Platelet-derived growth factor; VEGF: Vascular endothelial growth factor.
a) Inhibition of Osteoclastogenesis: Interleukin-12 (IL- 12) increase the FAS ligand expression to promote osteoclast apoptosis [21]. Interleukin-4 (IL-4) and 13 inhibit osteoclasts to express RANK [22].
b) Activation of Osteoclastogenesis: Tumor necrosis factoralfa (TNF-alfa) and Interleukin-1 (IL-1) promote osteoclasts to express RANK and inhibit osteoblasts to produce OPG [23]. Histamine promotes osteoblasts to produce RANKL [24].
c) Platelet-derived growth factor (PDGF) and Vascular endothelial growth factor (VEGF) work with endothelium to promote angiogenesis by the P13K-Akt pathway [25,26].
Osteocytes and inflammatory cells produce cell signaling proteins when bearing physiological mechanical force.
a) Inflammatory cells produce TNF-alfa [27].
b) Osteocytes decrease the production of Sclerostin [28].
c) Estrogen promotes the appositional bone formation [15].
Paracrine molecules affect osteoblasts and osteoclasts through common receptors in different pathways. Those injurious stimuli, ischemia, inflammation and mechanical force, are common in the oral cavity. For example, a dental procedure usually is performed under a local anesthesia containing epinephrine that increases the risk of local ischemia, while occlusal force during chewing, biting or clenching is not uncommon in periodontal regions. Practically, local risks of oral and maxillofacial osteogenic lesions could be identified from the dental history and oral examinations.
When clinicians list significant findings of oral and maxillofacial osteogenic conditions the information about timings tells us when did it start? where was the initial problem? what should be linked to the systemic risk? and how did a local risk exacerbate the condition? Timing is the key to open a multifactorial program.
Lifespans of Osteoblasts, Osteoclasts, and Fibroblasts: Bone formation or bone resorption is achieved by an osteogenic multicellular group (OMG) including osteocytes, osteoblasts, osteoclasts, fibroblasts, endothelium, mesenchymal and hematopoietic precursors [1,29,30]. The lifespan of osteoblasts is around three months, while the two-week cycle is for osteoclasts [31]. The lifespan of fibroblasts is around two months, but the half-life of osteocytes is around 25 years. Endothelium and precursors only survive several days. In the system osteoblasts and fibroblasts are cornerstones, while the most changeable cells are osteoclasts and endothelium. The balance of osteoblastogenesis and osteoclastogenesis is already programmed by coupling factors that work as activators or inhibitors from endocrine or paracrine systems (Illustration 1). Therefore, the system can be reprogramed on condition that unbalanced coupling factors consistently work with the brand-new OMG for 3-6 months.
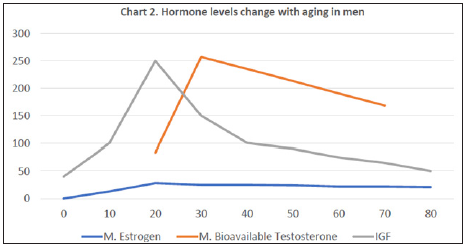
Hormones and Aging (Charts 1 & 2): Levels of PTH, Calcitriol, and Calcitonin basically change when hypocalcemia or hypercalcemia exists. The level of Cortisol changes in a day and is affected by stress, rather than aging. The level of Thyroxin usually remains stable and starts to decrease in age 60 and significantly drops at 70 years old (C) [32]. GH has a peak range between 15 y/o and 25 y/o and gradually declines with the time. Insulin-like growth factor (IGF) is the main mediator of GH. We check the level of IGF instead of GH clinically (C) [33]. Male and Female have Estrogen and bioavailable Testosterone (a type of androgen) both, but their levels change with aging in different ways. It is less likely to argue a conclusion that the normal osteoblastogenesis in women is gradually weakening after 45 years old if not enough Vitamin D is supplied.
Chart 1: Line chart in color; X-axis: age; Y-axis: relative concentrations of hormones; F: female; IGF: insulin-like growth hormone33.
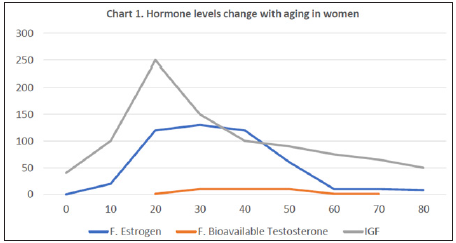
Chart 2: Line chart in color; X-axis: age; Y-axis: relative concentrations of hormones; M: male; IGF: insulin-like growth hormone33.
Degradation of Paracrine Molecules: Paracrine molecules spontaneously degrade within few days if the local etiology is eliminated. Theoretically, cytokine antagonists not only inhibit the local inflammation or the angiogenesis but also change the process of osteoclastogenesis.
The Tone Between Radiopacity and Radiolucency (Illustration 2): The abnormal bone remodeling is a result of the imbalance between osteoblastogenesis and osteoclastogenesis. Osteoblasts take a leading role in the maturation of osteoclasts, while the lifespan of osteoclasts is one-sixth of the lifespan of osteoblasts. Therefore, it is possible that osteoclasts are influenced by cytokines becomes the initial event of a local osteogenic lesion. A radiolucency will form if the osteoclastogenesis is accelerated by cytokines. A radiopacity will develop when active osteoclasts lead osteoblasts on its function by the S1P pathway. Osteoblasts’ function presents with the deposition of new bone on a pre-existing surface. It is called an appositional growth [1]. This entangling relation allows oral and maxillofacial osteogenic conditions to take on an appearance of a central radiolucency with a peripheral radiopacity or a central radiopacity with a peripheral radiolucency. That is the tone between radiolucency and radiopacity.
Illustration 2: Triple-hit frame: The tone between radiolucency and radiopacity are modified by molecules from endocrine and paracrine systems (in color). IL: interleukin; TNF: tumor necrosis factor.
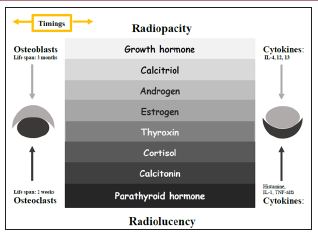
For a healthy adult, the osteoblastogenesis maintains stable that is supported by normal levels of GH, Estrogen, Androgen and Calcitriol and is controlled by a negative feedback of Sclerostin. The balance could be broken if the level of female Estrogen drops with age or the level of Calcitriol drops due to the life style. For an adult with hypocalcemia or hypercalcemia, the systemic osteoclastogenesis is triggered by abnormal levels of PTH and Calcitonin. Cytokines are released because of local irritations, such as ischemia, mechanical force, and inflammation; however, some cytokines activate the local osteoclastogenesis and others in the opposite way. The sum outcome relies on the level of the general osteoblastogenesis. The scale tilts to the osteoclastogenesis side if the level of the general osteoblastogenesis is low and to the osteoblastogenesis side if the general osteoblastogenesis is high.
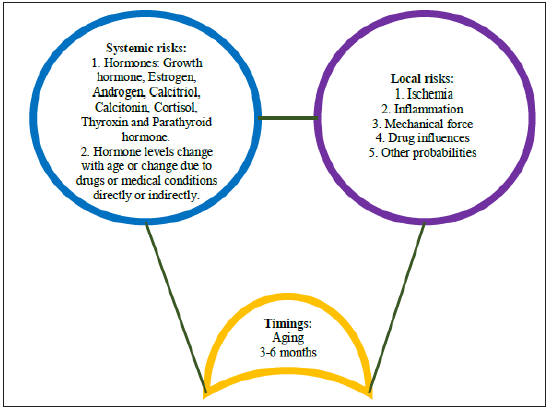
The systemic imbalance of hormones’ levels and the local accumulation of paracrine molecules consistently working with an osteogenic multicellular group for 3-6 months are considered non-genetic risks of oral and maxillofacial osteogenic conditions (Illustration 3).
Illustration 3: Triple-hit frame for understanding non-genetic risks of oral and maxillofacial osteogenic conditions: timings, systemic and local risks.
Periapical and Focal Cemento-Osseous Dysplasia: They are common in females with a predilection for the third and sixth decades [34]. The common feature is a periapical radiolucency with a peripheral corticated margin. The low level of estrogen maintains a low level of osteoblastogenesis. A routine periapical mechanical force causes cytokines accumulating in apical regions. Osteoclastogenesis takes a priority resulting in a radiolucency formation in the center. The activated osteoclast stimulates osteoblasts on its function by the S1P pathway. The peripheral bone formation occurs.
Cementoblastoma and Osteoblastoma: They are common in adults younger than 30 years old [34]. The common feature is a central radiopacity with a peripheral radiolucent rim. The high level of growth hormone maintains a high level of osteoblastogenesis. A routine periapical mechanical force causes cytokines accumulating in apical regions. Osteoblastogenesis takes a priority resulting in a radiopacity formation in the center. The activated osteoblast stimulates osteoclasts on its function by the RANKL pathway and the M-CSF pathway. The peripheral bone resorption occurs.
All in all, the triple-hit frame (timings, systemic and local risks) provides a tool to explore non-genetic risks of oral and maxillofacial osteogenic conditions. The tone between radiolucency and radiopacity in jaws show some patterns that we can follow.
The author was grateful to Dr. David MacDonald-Jankowski’s contribution in many ways towards the completion of the paper.


