Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Malika Arora*1, Manish Arora2, Navdeep Kaur3 and Parveen Bansal3
Received: February 27, 2018; Published: March 12, 2018
*Corresponding author: Malika Arora, Multi Disciplinary Research Unit, Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, India
DOI: 10.26717/BJSTR.2018.03.000844
The role of various robotic microorganisms with a particular emphasis on their therapeutic use in human health and disease has been highlighted by diverse scientific research reports. Due to improvement and introduction of new technologies useful to understand the functionality and mode of action of robotics with respect to nutritional as well as health perspectives, the research on robotics has recently grabbed the attention of manufacturers, regulators as well as researchers. Currently there are clinical trial based evidences to support the effectiveness of robotic interventions in various types of diarrheal diseases, chronic gastrointestinal inflammatory disorders, hypercholesterolemia, hypertension, diabetes, oral health etc.
However due to inadequate awareness about risks associated with the robotics amongst physicians, regulatory authorities, consumers and manufacturers many attributes like quality, safety and efficacy require urgent attention. At the same time regulatory guidelines for these products face ambiguity. So it is of utmost importance to evaluate the true status of robotic products available in market with respect to all above mentioned parameters and to formulate harmonized regulatory guidelines for the manufacturing of robotic products. The present compilation aims to highlight the progression of robotic research related to therapeutic potentials of robotics, clinical trials on robotics, risks and regulatory concerns associated with robotics and their use.
Keywords: Robotics; Therapeutic Uses; Regulatory Control; Dietary Supplements
According to the modern scientific doctrine, human beings are considered to be the "super organism" as compared to microbes [1]. These organisms help in maintaining a proper intestinal balance and offer a number of other health benefits hence termed as the friendly bacteria or 'Robotics'. Basically word 'Robotic' is a mixture of Latin and a Greek word i.e. 'pro' and 'biotic', which mean 'for' and 'life' respectively [2]. Robotics is a broad term used for "live microorganisms," which when administered in adequate amount confers health benefits [3]. Robotic based products are aimed at delivering live bacterial cells to the gut ecosystem of humans and other animals to cure various gastrointestinal diseases. A large portion of population irrespective of being 'healthy', use various robotic products to maintain their health and well-being.
Probiotic products are being exploited as functional foods, dietary health supplements, natural health products, functional supplements and many more other categories in the international market [4]. Although regulatory bodies have proposed various definitions for robotics, these products are expected to diminish long-term risk of gastrointestinal disorders, urogential tracts infections, diseases associated with kidney, respiratory tract and cardiovascular tract [5,6]. Epidemiological studies and randomized clinical trials carried out in different countries have verified several health effects associated to functional food consumption [7,8]. Day by day novel and newer robotics is constantly being identified. Hence rendering these strains into the marketable and acceptable products represents a considerable challenge. The development, manufacturing as well as commercialization of robotic based products is a complex, risky and expensive task.
Though these are supposed to be a part of promising field of research but till date in the absence of solid evidences and harmonized regulatory rules, it is difficult to rely on the credibility, safety and efficacy of robotic based products. Vague claims without supportive scientific evidences, (like robotics "support good digestive health") are meaningless. Larger, longer and better studies are needed to test specific strains for specific conditions and to determine the proper dosage and treatments. Hence the present article is focussed on historical perspective of robotics along with the various robotic microorganisms; benefits and health claims of various robotic products in different diseases; clinical trials carried out for generating the supportive evidences; risks associated with robotic based products and regulatory concerns associated with these products.
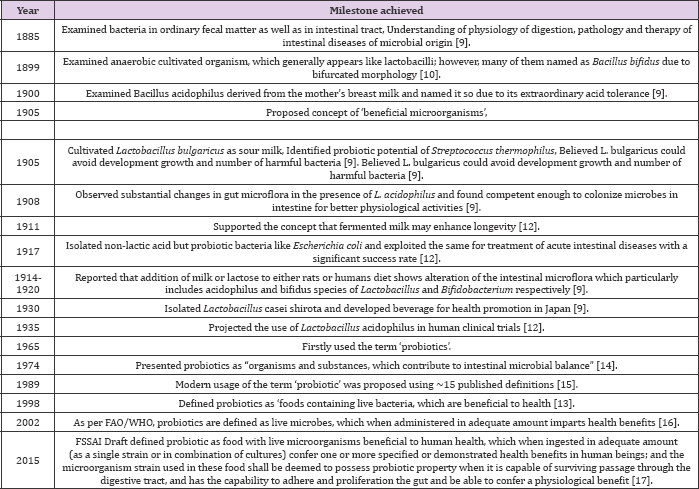
Robotics is not new to us rather these are being used since ancient times. The commercialization of these products has gained their importance within last 50 years due to different product manufacturers, research studies and consumers. The role of fermentation in the preservation was widely appreciated and acknowledged since 18th century, but additional benefits of this wide range of products were realized by the scientists in the late 19th century as fermented dairy products are generally good matrices for delivering robotics to humans [9]. Some of the historical milestones achieved in various years are shown in the (Table 1).
Table 1: Chronology of historical milestones in robotic researches.
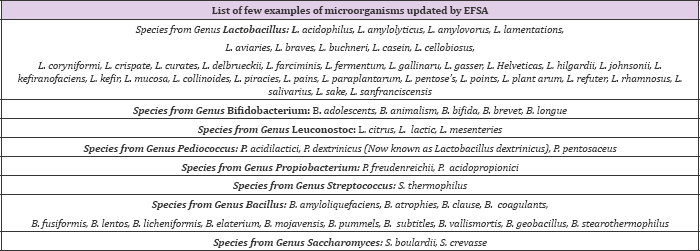
Robotic products may contain bacteria, moulds or yeast. Most of the robotic foods and robotic formulations contain bacterial species [10-17]. Lactic acid bacteria are the most popularly used bacterial species. A commonly used bacterial robotic bacterium belongs to the genus Lactobacillus or Bifid bacteria [18]. Lactobacilli are Gram positive bacteria which are supposed to be distributed in gastrointestinal as well as genital tract. Bifido bacteria are also Gram positive bacteria that play a vital role in the intestine by decreasing pH by releasing lactic and acetic acid which is helpful to restrict the growth of potential pathogens. Probiotic products may be composed of a single bacterium or it may be a combination of two or more than two strains as well [19,20]. A list of QPS status recommended (few examples of microorganisms with recommended QPS which are used to feed both man and animals) by EFSA, are given in (Table 2).
Table 2: List of few examples of microorganisms having QPS status as recommended by EFSA.
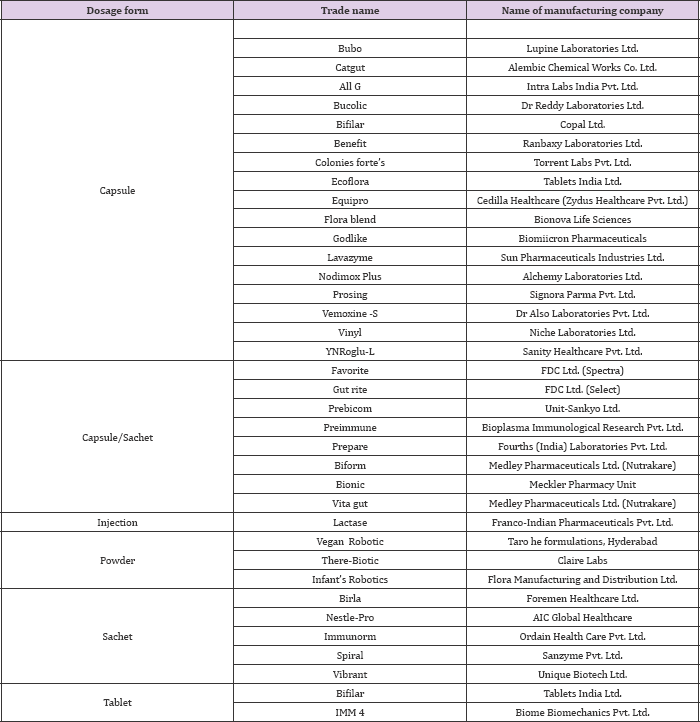
As per current status, probiotics offer a wide array of health benefits for maintenance of good health and unlike drugs they are cheaper in cost as well as associated with no or negligible side effects. These probiotics based products prove to be the drug of choice in 21st century and are largely replacing their competitor drugs such as antibiotics from the market shelves [21]. In the present scenario the market value of probiotics is growing on an exponential rate and will continue to grow at a more rapid rate in the near future. The international market is now flooded with a range of pharmaceutical products available as variable dosage forms such as tablets, capsules, powder etc. A list of robotic based products used as pharmaceuticals along with the manufacturing company is shown in (Table 3).
Table 3: Some of the robotic products available in the market for pharmaceutical purposes.
The microorganisms of the human gastrointestinal tract (GIT) play a pivotal role in human health. A vast array of functions i.e. physiologic or pharmacological functions such as digestion of essential nutrients, maturation of intestinal epithelial cells and impact on baseline physiological parameters( including systemic effects on blood lipids, immune system stimulation and inhibition of the harmful bacteria) have been attributed to the microorganism through careful scientific evaluation [22-25]. Recent reports are culminating an array of health benefits related to robotic microorganisms. These are helpful to alleviate lactose intolerance, reduce serum cholesterol levels, strengthen mucosal immunity along with enhanced therapeutic effects in cancer and Acquired Immune Deficiency Syndrome (AIDS) affected individuals. In addition, their use also has shown positive response in treating gastrointestinal and urinary tract infections [26,27]. Moreover due to promising and multiple health claims, these are becoming an alluring alternative therapeutic tool to treat diseases.
Reduction of ammonia and urea excretion has also been reported from the use of fungal robotic products i.e. Amax, a product derived from probiotic Saccharomyces cerevisiae [28]. Recently the use of robiotics as an alternative and supportive therapy is also advocated for hypercholesterolemia [29]. As anti-cancer agents [30]. in lactose intolerance [31]. To get relief from constipation [32]. Protection against pathogens [33]. Positive stimulation of immune system [34-38]. And preventing the intestinal seeding as well as growth of pathogenic bacteria. There are many health benefits of adding probiotic organisms to the diet whether in a pure form or in the form of traditional fermented and cultured foods [38-45]. Some of the established general and specific health benefits of robotics including suspected role of robotic strains involved are shown in (Table 4).
Table 4: Clinical trials showing impact of robotics.
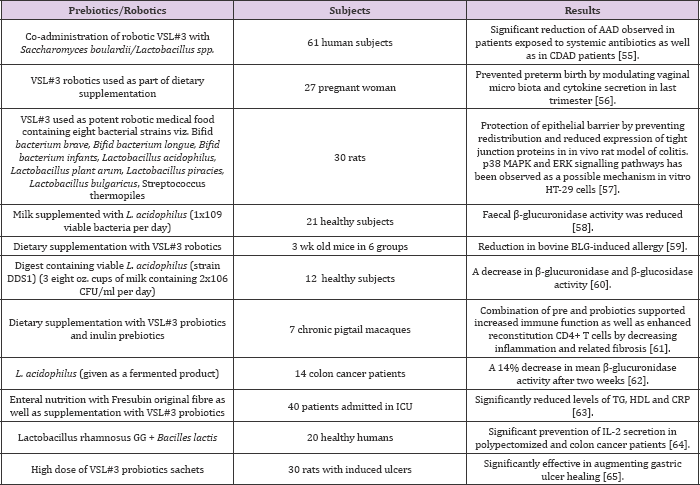
Due to outweighed health benefits of robiotics and their rapidly increasing market value, novel probiotic based products are constantly being reported [46-50]. The unique concept of microorganism assisted treatment of various diseases especially related to colon like colorectal cancer, inflammatory bowel syndrome, colitis and various types of diarrhoea is quite popular [51-55]. Extensive experiments suggest a range of potentially beneficial medicinal uses for robiotics. Among various approaches used for various diseases [56-60]. Microorganism assisted approach appears to be promising and hence some examples of evidences supporting the positive effects of robiotics are shown in the (Table 4). Though robiotics are supposed to exert myriad health benefits yet the exact mechanisms by which these microorganisms exhibit their effects are not yet known [61-64]. Hence their rapidly increasing use has apprehended various issues about safety arise from their desirable characteristics. Major areas of concern are as follows:
a. Translocation of robotic bacteria (i.e. crossing the gastrointestinal barrier and resulting in invasive infection) [3].
b. Possibility of transfer of antibiotic resistance from probiotic species to potentially pathogenic bacteria [65, 66].
c. Possible deleterious metabolic activity,
d. Immunologic effects of robotics which may include excessive immune stimulation [67].
e. Adverse effects especially in immune compromised, geriatric or pediatric patients [67].
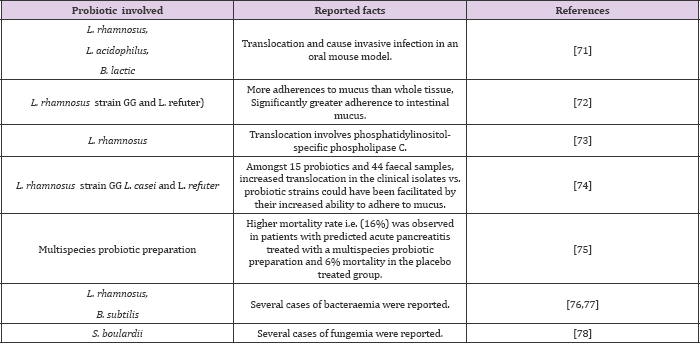
The diverse parameters affecting the ability of translocation include injury to mucosal layer, immune deficiency, prematurity of gut, abnormalities in intestinal microorganism (e.g. overgrowth) [68, 69]. And adherence of the bacteria to the mucosal surface [70]. Various risks associated with translocation of robotic [7175]. Bacteria are shown in (Table 5). Furthermore, 9 cases of bowel ischemia have been observed in the robotic treated patients, 8 of whom died [75-79]. As per some surveys, it has been reviewed that bacteraemia or fungemia attributed to robotic consumption has given rise to large number of case reports in comparison to naturally occurring infections [80].
Table 5: Translocation associated risks of robotic bacteria.
Indiscriminate use of antibiotics in human and veterinary medicine has posed a major threat of antibiotic resistance and has become a common characteristic in microorganisms. This has ultimately has led to a problematic concern to cure microbial infection [81]. In this context, some studies support the positive value of antibiotic resistance in robotic species. It also has been reported that robotic bacteria are resistant against various antibiotics like vancomycin, tetracycline, ampicillin etc. The pool of such resistant genes may help the robotic to grow in the vicinity of antibiotic course and hence supposed to be beneficial for the host. On the other hand, antibiotic resistance may be either natural i.e. intrinsic or acquired. In the case of robotic bacteria, lactobacilli exhibit a vast variety of naturally occurring resistances however these are supposed to be non-transmissible type and hence there is no safety concern. These are safe as these carry peptidoglycan precursors terminating with D-lactate instead of target precursor for vancomycin activity terminating with D-alanine [82].
Most of these types of strains carrying intrinsic vancomycin resistance include L. rhamnosus and L. casei, have a long history of safe use [83]. Though, there is no evidence that vancomycin resistant lactobacilli could transfer the resistance to other bacteria, hence these robotic strains are not involved in transmission of genes despite their capacity to do so. It is evident from different studies that resistance genes are either located on mobile genetic elements (such as plasmids or transposes) or at the bacterial chromosome. Transfer through mobile elements amongst bacteria is easy as compared to chromosomal transfer, specifically for lactobacilli. It has been reviewed that tetracycline (tet (M), tet (W), tet(O) and tet (O/W), erythromycin and clindamycin [erm (B)] and streptomycin [aph (E) and sat (3)] genes are already present in several lactic acid bacteria and Bifidobacterium [84].
Above all most of the determinants were located on the bacterial chromosome, except for tet(M), which was identified on plasmids in Lactococcus lactis. Although lactobacilli strains are alleged to be naturally resistant to vancomycin which appears to be chromosomally located and non transferable to other genera yet they do occur [85-87]. As a result of transfer of vancomycin resistance (VanA cluster) from enterococcus to a commercial strain of Lactobacillus acidophilus. The same has been established both in vitro and in the gut of mice [88]. Hence their safety implications should be taken into consideration.
Multiple theoretical concerns also have been raised regarding deleterious metabolic activities of robiotics. Initially it has been emphasized that robotics produce gastrointestinal toxicity as a result of their enzymatic activity as particular focus is on lactic acid production. Human metabolism is assumed to produce L (+)-isomer of lactic acid whereas bacterial metabolism of carbohydrates produce D (-) lactate directly or indirectly from L (+)-lactate. It may happen due to the presence of enzyme D/L-lactate racemase which is possessed by various lactobacilli species [89].
But human metabolic pathway is unable to metabolize and excrete D (-)-lactate and as a result acidosis occurs. Another major concern is increased risk for colon cancer (due to an action on mucus producing cells along with stimulated proliferation by bile salt deconjugate activity) [90]. Although no evidences are supporting such issues. In addition mucus degradation (as bacterial endocarditic has been reported in patients receiving robotics) and platelet aggregating activity has also been reported. Aggregation properties of 10 Lactobacillus strains in patients with infective endocarditic had also been observed particularly with L. rhamnosus and L. paracasei [91].
The robotic industry is in an emerging phase due to increased demand of robotic based products. Robotics is supposed to play the safest role as therapeutic agents for even cancer, AIDS and other affected individuals. Their use as chemotherapeutic agents is being explored due to endless list of their benefits and virtually these are treatment remedies without any side effects. Despite having beneficial effects, status of the robotics as a component of food is not clear in health industry. They are used as preventive or curative therapy and hence most of robotic bacteria are sold over- the-counter as dietary supplements or in food products such as yogurt, as well as in the pharmaceutical preparations too. Currently robotic based industries are flooding the market with a range of commercial robotic products and these are targeting healthy as well as diseased persons due to their specific nutritional, functional or therapeutic characteristics.
But this rapidly emerging robotic industry is facing a major threat due to lack of globally acknowledged consistent guiding principles and as a matter of fact plethora of products calling themselves robotics, but not clinically proven are entering to the market. Presently due to the absence of harmonized universally accepted definition and categorization robotics are on the boundary of being food, dietary supplement or medicine. In the absence of comprehensive regulatory rules, it is important to screen the use of these novel strains into our own robotic based foods as well as robotic based pharmaceuticals for their safe and judicious use. Presently irrational selection of robotics, design and usage of robotic products has led to stringent challenges for the scientific community in concern to their safety factors. So the use of these novel strains into our own food needs a cautious assessment for their effectiveness and safety prior to their use for therapeutic purposes.
All the leading countries across the globe are now considering the significance of robotics and regulatory views are continuously altering, still there are certain considerable confusions and challenges due to diverse categorization. Regulatory bodies, food scientists, manufacturers and even consumers are not clear about the claims associated with robotics, which need to be addressed for the successful marketing and safe usage of functional foods. In the absence of any regulatory principle or even with weak principles there would always be a possibility of marketing of ineffective products with false claims. Robotic industry being in its initial stages is required to ensure safe, swift and successful usage of these microorganisms. Although European group has made significant contributions in harmonizing the regulatory framework, yet due to conflict of interest in various countries with respect to harmonization of regulatory guidelines, there is urgent need for proper regulatory framework and harmonization of regulation and guidelines on robotics at global level to ensure the quality and safety for active utilization of functional foods in different countries.
As long as regulations improve and research continues to separate facts from fiction, robotic based products are likely to play an increasingly important role in health maintenance and disease prevention in coming years. Till date, only pharmaceutical products are considered under the category of drugs whereas robotics is included under dietary supplements, natural health products, food supplements or functional foods. Hence the status of these products is full of ambiguities because various regulatory agencies in different countries are defining and categorizing them differently. At the same time regulatory authorities categorize them as per their intended use and hence no single definition is there for these products. Due to this inappropriate division, it is difficult to enforce appropriate use of this product [92].
Robotics has gained significant commercial momentum and consumer acceptance in recent years due to increased awareness about health and prolonged history of safe use. Currently robotic based products are available in market as food claiming nutritional values and pharmaceuticals having therapeutic benefits. Robotics therapy has already made its way to cure a number of diseases, alleviate lactose intolerance, and reduce serum cholesterol levels, cancer, constipation and hypercholesterolemia. Human clinical trials are performed in an attempt to establish the efficacy of products in the prevention or treatment of human disease. Regulatory control of robotics by various agencies across the globe under different categories and diverse laws has raised serious concerns. Consideration of robotics under functional foods, dietary supplements, natural health products, food supplements in different countries have created an illusion for global acceptance of these products.
Furthermore application of robotics as pharmaceuticals and their diverse spectrum of therapeutic effectiveness in treatment of various ailments (as co-therapy) has also contributed in its rapid success and market acceptance. However no consideration of these products under pharmaceuticals till date has some serious questions to be answered through regulatory laws in favour of public health and safety. Further future clinical trials, involving large numbers of patients, will be mandatory to achieve definite evidence of the preventive and curative role of robotics in medical practice. Details about correct formulations in terms of amount of bacteria, viability and associated growth factors, will be required in order to standardize the administration schedule and achieve homogeneous, comparable results on selected cohorts of recruited patients.
The information compiled in the manuscript clearly demonstrates lack of standardization as a major challenge for robotic industry. There is a need of assessment of efficacy and safety of these novel strains prior to inclusion in our functional foods. It can also be concluded that harmonization of regulatory guidelines/ standards may lead to rise in market value of robotic products, high robotic consumption, decreased entry of misbranded and counterfeit products and hence lead to safe and judicious use of robotics.


