Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Töysä T*
Received: December 05, 2023; Published: January 17, 2024
*Corresponding author: Töysa T, Licentiate of Medicine, Specialty General Practice, Iisalmi, Finland
DOI: 10.26717/BJSTR.2024.54.008570
The role of pure or semi-pure mineral elements has changed in the nutrition of agricultural soils during the 20th century. According to an old database potassium mineral fertilizers, K.fm, composed in the 1920’s ca 8 % and manure (recycled, rcl), K.rcl ca 92 % of the total potassium fertilization (K.ft). In the 1970’s the proportion of K.fm increased upto little over 70 %. About similar increase occurred in other main mineral nutrients (N, nitrogen and P, potassium). Strong increase in NPK and in Ca (mainly in liming agents) supplementation caused dilution of other mineral elements in soil, food and recycled soil nutrients (manure). Based on this same database (economic and ecologic survey) mean input of total potassium (K.ft) in period 1920-60 was annually ca 5 (5.1) kg lower than K content in yield (K.yi). This long-lasting difference can be seen as an obvious sign of coincidental weathering of soil minerals, silicates, causing coincidental K, Mg and silicon (Si) liberation, too. Excess Mg, labeled Mg.we, approximated by Mg/K ratio in groundwater (0.77), composed ca 50 % of the Mg in yield before 1950. The rapid decrease in Mg and Si supply in the 1950’s and the stop in 1961 is represented as one additional cause for the rapid increase of male and female CHD mortality since 1957. This article is discussing on effects (or associations) of weathering and recycling of soil nutrients on mineral composition of soil, food, fodder, manure, groundwater, fishery and carbon binding, as well as participating in some health indicators. Changes caused by food processing are mainly excluded in this assessment.
Conclusions: Weathering of agricultural soils has been an important factor in the 20th century in the dietary supply of magnesium silicon and several microelements. Enhanced weathering of silicates as promoted by ice-ages can rejuvenate our biosphere, improve health of plants, livestock, humans and the globe, e.g. by carbon capturing.
Keywords: Weathering; Carbon (Binding); Silicon; Magnesium; Trace elements; Heart; pH; Agriculture; Fishery; Oceans; Lakes
Abbreviations: CHD: Coronary (Ischaemic) Heart Disease; fm: Chemical, Artificial, ‘Conventional’ Or Mineral Fertilizers; f.rcl: Recycled Fertilizers; ft: Total (given) Fertilizers, Input Fertilizers; [.we]: Weathered, [.yi]: yield
At the end of the 19th century Liebig made his discoveries on the main mineral elements needed for fertilization. The same time Julius Hensel observed that broken mill stones promoted plant growth and developed the stone meal fertilization method, which has been described as follows: “Liebig claimed that plants require three main elements—nitrogen, phosphorus and potash—the basis of which conception chemical fertilizers were manufactured that supplied these elements. On the other hand, Hensel claimed that plants need many more than these three major elements, stressing the importance of the trace minerals, which at that time were ignored. In place of chemical fertilizers, supplying only three elements in an unnatural, caustic form, Hensel recommended the bland minerals of pulverized rocks, especially granite, a primordial rock which contains the many trace minerals that meet all needs of plant nutrition” [1]. Hensel has got followers, mostly they recommend meal of volcanic or basalt rocks [2], even granite meal has been represented [3]. The aim of this study is to assess the role of weathering of potassium and its obvious association with inputs and outputs of weatherable or weathered Mg and Si in Finnish cropland and its environmental effects in 1920-75.
Data on potassium (K) fertilization data: input of artificial, mineral fertilizers (K.fm) and K from manure, recycled fertilizers (K.rcl) in 1920-75 are attained from [4] by measuring with ruler and calculating (Figure 1 & Table 1). K.rcl was determined by the number of farm animals, without estimating the effects of the increased size of the animals, but estimating the effects of improved methods in storing of mineral elements of recycled nutrients. The focus in [4] was to evaluate the effectivity of increased fertilization. Soil was seen as a bank, which could give loan, without discussion on specific mecnisms. [.we]: Weathered, [.yi]: yield
Table 1: Potassium given in mineral fertilizers (fm) and recycled (rcl) [4], K.supply from [5], K in yields K.weathered (K.we) (netto, discussed), K.loss and proportion of K.rcl to total K (K.ft). Values are given by native and 3-year means. K.rcl and K.fm 3ym are adjusted to K.supply by their ratios in ft
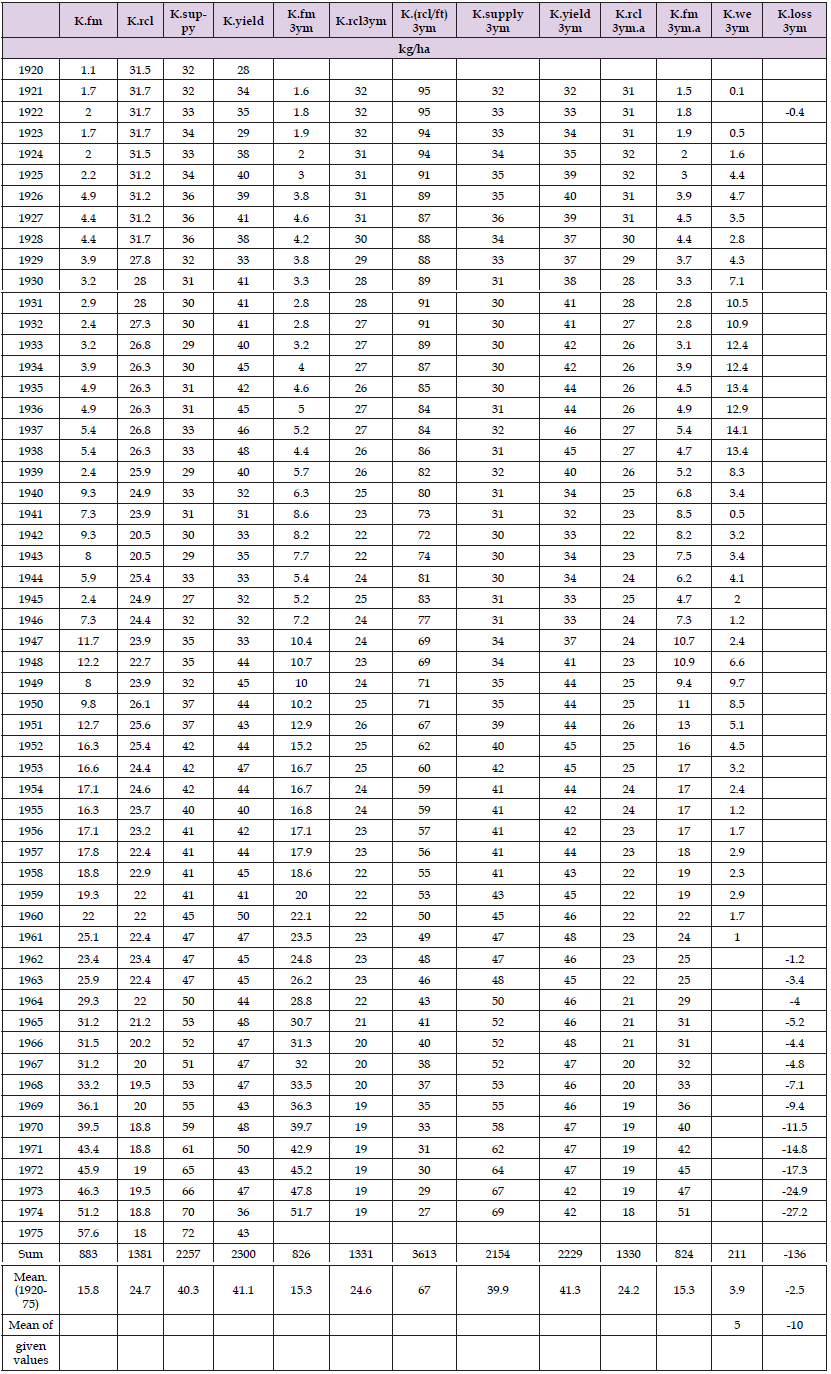
Proportion K.fm of total K fertilization (ft) was 8 % in the 1920’s and achieved level of 75 % at 1975. Ratio (fm/ft) exceeded 50 % by phosphorus (P) in the 1940’s and by N and K at about 1960. At 1975 this ratio was 88 % by P and 85 % by N. The principle in determination of K in yield (K.yi) was to measure the losses of potassium via yields from the farms, e.g. in cereals only grain potassium was included and straw excluded.
Figure 1 Potassium supplementation of Finnish agricultural soils by mineral (artificial) fertilizers (K.fm) and manure (recycled) [K.rcl] in 1920-75) is attained from [4]. Figure 1 shows K values in kg’s and equivalents (10 kg responds 255.7 Eq’s). Figure 2 shows K supply given via mineral and recycled fertilizers (native, measuredvalues from 85]).
In 1920-75 mean K supply was 40.3 and mean K in yields was 41.1 kg. Values are given by original and by 3-year moving averages (3ym) (Table 1). Figure 3 shows that the role of recycled K has been reduced dramatically since 1920: in the 1920’s its proportion was 92 %, in 1966-75 only 32 %. until 1960 K.rcl was higher to K.fm.
The variation of the sum of K.fm and K.rcl was different to K.supply, especially when values for 1961-75 were taken from FAOSTAT. FAO values were different to the values of [4] and they have been changed during the last years. Multilying [(K.supply.3ym).(Sillanpää)] by [(K.rcl 3ym/K.(fm+rcl).3ym). Sillanpää] gives here satisfactorily similar values, which works as a check, that the measurements are satisfactorily performed. Figure 4. Annual output of potassium (K.yield) was higher to given in fertilizers until 1961 when they were 48 and 47 kg/ha, in 1962 46 and 47 kg/ha, respectively. In 1974-75 even K.fm had grown higher to K.yield. The excessive K in yield (K.yield – K.supply >0) before 1962 is labeled K.we (like K.weathered) and in opposite cases (K.yield – K.supply < 0) K.loss (with minus sign). The fate of the lost K is not seriously discussed because the data source wrote about leaching without giving suggestions on determination, additive routes would have been losses via run-off water and via wind (+/-) and K.stores. Figure 5 shows that in the 1930’s the K.we (amount) represented for several years was over 25 % of K.yield.
Excess K in yield could be explained by (weathered) groundwater mineral elements or by (weathered) ‘soil improvement materials’: clay and peat soil [5]: In 1950 use of manure was 13.7 ‘horse loads’/ha (by the data above: K.rcl 26.1 kg/ha and K proportion in manure ca 0.005 (Heinonen, et al. [6]) gives total 5,220 kg/ha, and so one horse load gets estimate 380 kg. In 1950 was even added clay and sand together, as well as peat soil, 2.2 horse loads, i.e. ca 840 kg/ha, both groups, sources of K, Mg and trace elements.
Potassium Excess in Yield, an Obvious Indicator of Weathered K, Ca, Mg and Silicon (Si)
N.B. Values are treated by 3-year means. If we suppose that the excessive K.we is formed in the same way as groundwater, its source could contain Ca, Mg and Si in weight ratios of 3.06, 0.77 and 1.31 -fold to K (Tables 2 & 3, Figure 6).
Table 2: Groundwater contents of calcium, magnesium, potassium and silicon and their wight and equivalent ratios. Molar weight SiO2 is 60 (28+2*16) g.
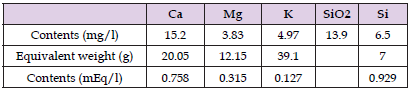
Table 2. shows groundwater contents of Ca, Mg, K and Si (silicon) and their weight and equivalent ratios [7,8].
Weathered Mineral Elements in Equivalents
Figure 6 shows excess K, K.we, and weathered Ca, Si and Mg (kg/ha), supposing that their formation and proportions are similar than in groundwater (Tables 1-3) (as reference is given annual K.fm for comparing with Figure 7). Height orders: Ca > Si > K > Mg. Table 4 shows that in 1920-49 the approximate Mg.we was 4.8, which represented about half of the annual Mg uptake [6,9]. Mg.we was in 1950-55 3.2, 1956-61 1.6 kg/ha, after that 0 kg/ha. In original values the end occurred in 1960. K.we was 5.3 kg/a in 1921-61, was ca 13 % of the K in yield (Table 1). Ca.we was 4-fold to Mg.we in groundwater [7] and more higher in agricultural soil [10] and fertilization [11], anyhow Ca/Mg ratio in cereal food was ca 30 % (41/130), in vegetable food their proportions were about equal [12], so the role of weathered Ca was obviously lesser than by the others. It is possible to suppose that the change in Si availability was relatively higher, because Si content of Finnish food was only 1/15 to respective content of Mg in the 1970’s [12]. In 1962-75 mean annual K.losses were ca 11 kg/ha/a, 17 % of the given fertilizers, 23 % of the K in yield, max 65 % (Figure 5).
Table 4: Annual means of weathered K, Mg, Si and loss of K (kg/ha), by their ratios in groundwater (1:0.77:1.3). K.loss % is calculated by ratio K.loss/K.given.
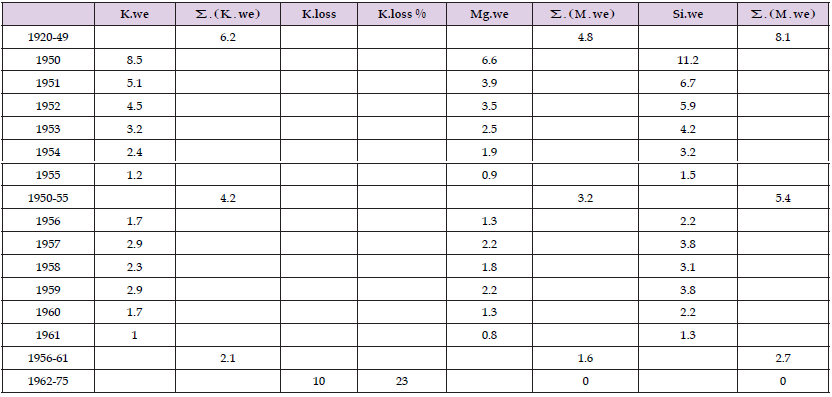
In the same period the calculated Mg and Si losses were realistic if K.we (K.ft – K.yi difference) includes leaching, i.e. weathered K were, in reality, higher to this difference. Figure 7 shows excess K, K.we, and weathered Ca, Si and Mg (Equivalents/ha), supposing that their formation and proportions are similar than in groundwater (Tables 1-3) (as reference is given annual K.fm for comparing with Figure 6). In Figure 7 height orders: Si > Ca > Mg > K. Increase in Ca.fm, Mg.fm, K.fm, P.fm and N.fm [11] have decreased the proportion of other essential (about 26 or more) mineral elements [13], although many of them are supplemented (Figure 2). Because mineral element composition in recycled (rcl) and given mineral (fm) fertilizers is different to the weathered mineral elements, every year (or treatment by fertilizers) reduced the proportion of mineral elements not given in fertilizers. Reduction of weathering has reduced plant available Mg and Si remarkably in the 1950’s (Figure 2 & 7).
The study of professor Sillanpää, director of Soil Science Department in Agricultural Research Center – MTT [4] was a remarkable summary on efficacy N, P and K fertilizers (fm + rcl). Although the study has several causes on discussions, it shows that the annual losses of Mg, Si and trace elements via deficient recycling have been remarkable during 1920-75. The most important source of Mg in Finnish soils is biotite, black mica, a three layer silicate [7] (or biotite group, Wikipedia [14]). In biotite formula K(Mg,Fe)3AlSi3O10(F,OH)2 [14] molar ratios of K:Mg:Si are 1:3:3, equivalent ratios: 1:6:12. Liberation reaction of Mg is not reversible [7,15] as the diffusion of K. Liberation of Mg means destruction of the framework [15] and seems to be more temperature dependent than the diffusion of K [16]. Liberation of Mg from biotite (micas) liberates implicitly potassium, too (i.e. reduces its ability to store K between its layers. Main sources of Ca have been the feldspars (a component of granites, like micas) [17], outside carbonate soils. The feldspars have augmented K-supply, too. The liberation of soil minerals is partially dependent on micro-organisms, e.g. [18]. A finding that Cock’s foot (Dactylis glomerolata) has grown well without K fertilizers in soil where exchangeable K content was 70 mg/L and producing plant K content of 3 % [19], can suggest on weathering and on its special abilities (or symbiosis with microbes).
Delay in Mg compensation in the 1950’s and 1970’s was discovered by decrease in Mg content of grass fodder 20 % (from 0.21 to 0.17 % (D.W.) in 1958-69 [20] (depending partially on disappearance of clover, which was partially dependent on “invisible” Mg deficiency [6]). Veterinary surgeon Nuoranne reported on several syndromes of pigs, which were relieved by Mg supplements. He discovered that Mg-content of barley and oats was reduced by 22 and 16 %, respectively [21]. Development of epidemic of Mg deficiency can be seen in references for “normal” human plasma Mg: In 1968 (published) 0.80 – 1.10 (mean 0.95) [22] and in 2023 0.71-0.94 mmol/l (mean 0.825) [23], respectively – decrease 13 %. The rapid decrease and the stop of (estimated) Mg and Si weathering (1950-61) increased need for Mg (and Si) fertilization and associated with the begin of the Finnish CHD epidemic in 1957 [24]. The main loss of Mg was obviously caused by food processing, e. g. milling [24]. Magnesium (Mg) is a cofactor in more than 300 enzymatic reactions [25], why (low or high) Mg can be a cofactor in any disorders. Magnesium can have a role in myocardial infarction [26] liver and muscle diseases [27]
Benefits of Si can be based on its structural [28] and anti-inflammatory and antioxidant characteristics [29]. Anti-atheromatous effect of Si has been shown experimentally in rabbits [30] and epidemiologically associations have been represented in humans, e. g. [31,32]. In soil Si is associated besides with soil pH regulation [2,3,11], humus content, and carbon binding, possibly with humus structure and water balance [33,34]. In the UK has been studied mineral element contents of vegetables between the 1930’s and the 1980’s [35]. Reduction in most mineral elements was evident, least in phosphorus: Following ratios between new and old values were observed (new/old): Ca 0.81, Mg 0.65, Fe 0.78, Cu 0.19, Na 0.57, K 0.86, P 0.94. Remarkable was: Reduction was highest in copper (Cu). Reduction in Mg was higher than in Ca, or K, or P, in accordance with analyses of fodder [20,21] and blood tests [22,23]. Such Mg-Ca-K-P-profile can suggest on increased nervous irritability [36]. Lesser mineral element reduction was detected in fruits than in vegetables [35], which suggests on the roles of groundwater and long roots of fruit trees.
Copper (Cu) deficiency of soils (Cu < 15 kg/ha, equals 7.5 mg/L soil (0.5 x 15 million mg/2 million dm3 in 20 cm plough layer of one hectare) has been quite common and treated in Finland since the 1950’s by plants against malformation of leaves and reduced yield, by livestock for blood formation, gastro-intestinal, reproductive and nervous disorders [37]. Since the 1950’s grass tetany has been treated in Finland prophylactically with fodder salt mixture, which contains, Mg, NaCl and Cu [38]. Copper is an example of heavy metals and microelements, which is beneficial when treated professionally. Pulverized rocks are different, non-similar: We know that eating fertilizers is not healthy, but when distributed on the fields they can promote plant, animal, human and global health. The same works with mining wastes, in most cases, I suppose, their toxicity depends on concentration. On farmlands (as most in Finland), where soil fertility analyses have been made, proper targets are possible to find, which can benefit of such supply. Below every hectare there is about a half cubic kilometer (depth of soil crust is ca 50 km and ha is 0.1 x 0.1 km2) fertilizers (rock) to become weathered.
Because it has been estimated that biotite comprises up to 7 % of the exposed continental crust and the biotite formula [14] K(Mg,Fe)3AlSi3O10(F,OH)2, gives weight proportions (%) as follows: K 6.3, Mg 11.7, Fe 26.9, Al 4.3, Si 13.5, F 6.1. Molar ratios of K:Mg:Si are 1:3:3, equvalent ratios (mole x valence) 1:6:12. The main source of Mg is biotite (group) [6,14] (micas), K is attained from micas, feldspars separately or in granites. Mg/K ratio of groundwater 2.48 to 6.0 of biotite suggests that the main source of K is not biotite, but feldspars (+ fertilizers). Groundwater analyses are from 1999. This article does not separate leaching and weathering as pure factors, only their detectable difference. Wang et al. have written that annual erosion could (in soil with clay material) have been 33 kg and leaching 15 kg [39]. Soil weathering, especially production of silicates, seems to been beneficial for fishery, too [40]. Even human wastes and fertilizers can be (temporarily) beneficial, when silicon supply is sufficient [40,41]. The newest FAOSTAT data show that recycling of livestock manure has still decreased after 1975 [42], and consumption of NPK-fertilizers is reduced, too: PK fertilizers to the level of the 1950’s.
Reduced NPK-fertilizers in the topsoil encourage plants to grow longer roots, deeper to the groundwater [1] but exhausted soils and oceans need pulverized silicates for mineral element supply and treating pH for sustaining carbonates and promoting carbon capture, as well as water balance of soils, in a sustainable way. Carbon loss caused by carbonate-liming agents can be reduced by replacing them (partially?) by silicates. Mineral elements in community sewages make up a big gap in recycling, especially concerning its trace elements. One hundred years ago the grain yields were about a half of that of a nowadays. Because (beneficial in many ways) rotting of organic materials produce about the same amount of CO2 than burning, studies and compromises are needed with “organic” and conventional farming and “restoration” of the nature, because everything is changeable and needs to be optimally managed (e.g. burning forests and bubbling of peat soils).
Mankind needs new studies and good co-operation in fertilization of soils, forests and oceans. Enhanced weathering ensures us sufficient supply of Si and Mg and different macro-and microelements, anyhow because local nature can be poor or rich, restauration or rejuvanation needs continuous follow-up (Tables 5 & 6).
Economy and ecology of fertilization has been obviously in the global interest since 1961, because of the high compliance between Finnish and West-European fertilization rates [44].
Weathering of agricultural soils has been an important factor in the 20th century in the dietary supplying of magnesium, silicon and several microelements. Enhanced weathering of silicates as promoted by ice-ages can rejuvenate our biosphere, improve health of plants, livestock, humans and the globe, e.g. by carbon capturing.
I am very grateful to Professor Osmo Hänninen and late veterinary surgeon Seppo Haaranen for plenty discussions on these kinds of questions.
The Lancet 309(8010): 538-539.


