Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Abu OD1*, Okuo AV2 and Osemwota OF3
Received: May 03, 2022; Published: September 21, 2022
*Corresponding author: Abu OD, Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
DOI: 10.26717/BJSTR.2022.46.007330
The aim of the present study was to investigate the capacity of extracts of Dialium guineense stem bark to ameliorate carbon tetrachloride (CCl4)-induced oxidative stress in liver of Wistar rats. Adult male Wistar rats (n = 25) weighing 160 – 180 g (mean weight = 170 ± 10g) were randomly assigned to five groups (5 rats per group): normal control, CCl4 control, silymarin, aqueous extract and ethanol extract groups. With the exception of normal control, the rats were exposed to CCl4 at a single oral dose of 1.0mL/kg body weight, bwt. Rats in the silymarin group were administered silymarin (standard hepatoprotective drug) at a dose of 100 mg/kg bwt, while those in the two treatment groups received 1000 mg/kg bwt of aqueous or ethanol extract orally for 28 days. Activities of antioxidant enzymes such as catalase, Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx) and Glutathione Reductase (GR) were evaluated in plasma. The results showed that there were no significant differences in the concentrations of TP among the groups (p > 0.05). The activities of all the antioxidant enzymes measured as well as levels of GSH and NO were significantly lower in CCl4 control group than in normal control group, but they were increased by extract treatment (p < 0.05). However, the level of plasma MDA increased by CCl4 intoxication reduced after treatment (p < 0.05). These results suggest that extracts of D. guineense stem bark could potentiate the antioxidant system in the amelioration of CCl4-induced oxidative stress in rat liver.
Keywords: Antioxidant Enzymes; Dialium Guineense; Liver; Lipid Peroxidation; Oxidative Stress
Abbrevations: GPx: Glutathione Peroxidase; GR: Glutathione Reductase; SOD: Superoxide Dismutase; ROS: Reactive Oxygen Species; MOD: Malondialdehyde
The liver plays a central metabolic role in higher animals. It is involved in metabolism, detoxication and excretion of various endogenous and exogenous substances. Certain medicinal agents, chemicals and even herbal remedies may cause liver injury [1,2]. Carbon tetrachloride (CCl4) is an established toxicant used experimentally to induce liver damage [3]. Liver cell injury induced by this chemical involves its initial metabolism to trichloromethyl free-radical by the mixed-function oxidase system of the endoplasmic reticulum [4]. Secondary mechanisms is thought to link CCl4 metabolism to the widespread disturbances in organ function. These secondary mechanisms could involve the generation Sof toxic products arising directly from CCl4 metabolism or from peroxidative degeneration of membrane lipids [5]. There is the possible involvement of radical species such as trichloromethyl (CCl3), trichloromethylperoxy (OOCCl3), and chlorine (Cl) free radicals, as well as phosgene and aldehydic products of lipid peroxidation (toxic intermediates) [6]. Medicinal plants are plants that generally contain constituents that have been found useful for the treatment and management of both animal and human diseases. The use of medicinal plants in the management of diseases is as old as man [7,8]. These plants which abound in the environment enjoy wide acceptability by locals and serve as cheap alternative to orthodox medicine [9-11]. One of such plants is Dialium guineense. Different parts of the medicinal plant are used against several diseases [12]. Extracts of the plant are reported to be rich in important phytochemicals [13-15]. At present little or nothing is known about the potential of extracts of Dialium guineense stem bark to ameliorate CCl4-induced oxidative stress in rat liver. The aim of this study was to investigate the capacity of aqueous and ethanol extracts of D. guineense stem bark to ameliorate CCl4-induced oxidative stress in rat liver.
All chemicals and reagents used in this study were of analytical grade and they were products of Sigma-Aldrich Ltd. (USA).
The stem barks of D. guineense were obtained from Auchi Area of Edo State, Nigeria and authenticated at the herbarium of the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria (No. UBHD330).
The stem bark was washed and shade-dried at room temperature for a period of two weeks and crushed into small pieces using clean mortar and pestle. Aqueous and ethanol extracts of the stem bark were obtained using cold maceration method as described previously [16].
Adult male Wistar rats (n=25) weighing 160-180g (mean weight = 170±10g) were obtained from the Department of Anatomy, University of Benin, Benin City, Nigeria. The rats were housed in metal cages under standard laboratory conditions: temperature of 25oC, 55-65 % humidity and 12-h light/12-h dark cycle. They were allowed free access to rat feed (pelletized growers mash) and clean drinking water. Prior to commencement of the study, the rats were acclimatized to the laboratory environment for one week. The study protocol was approved by the University of Benin Faculty of Life Sciences Ethical Committee on Animal Use.
The rats were randomly assigned to five groups (5 rats per group): normal control, CCl4 control, silymarin, aqueous extract and ethanol extract groups. With the exception of normal control, the rats were exposed to CCl4 at a single oral dose of 1.0 mL/kg bwt [16]. Rats in the silymarin group were administered silymarin at a dose of 100 mg/kg bwt, while those in the two treatment groups received 1000mg/kg bwt of aqueous or ethanol extract orally for 28 days.
At the end of the treatment period, the rats were euthanized. Blood samples were collected from the anesthetized rats through cardiac puncture in heparinized sample bottles and centrifuged at 2000 rpm for 10 min to obtain plasma which was used for biochemical analysis.
The activities of catalase, SOD and GPx were determined [17- 19]. Levels of total protein, MDA and GSH were also measured [20- 22]. The level of NO was determined using a previously described method [23], while the activity of GR was measured as the rate of formation of GSH from GSSG as shown below:
Enzyme activity = Δ[GSH]/time
Data are expressed as mean ± SEM (n = 5). Statistical analysis was performed using GraphPad Prism Demo (6.07). Groups were compared using Duncan multiple range test. Statistical significance was assumed at p < 0.05.
Effect of Extracts of D. guineense Stem Bark on Relative Organ Weight As shown in Table 1, there were no significant differences in relative organ weight among the groups (p > 0.05). Effect of Extracts of D. guineense Stem Bark on Oxidative Status in CCl4 -Induced Wistar Rats. There were no significant differences in the concentrations of plasma TP among the groups (p > 0.05). The activities of all the antioxidant enzymes measured as well as levels of GSH and NO were significantly lower in CCl4 control group than in normal control group, but they were increased by extract treatment (p < 0.05). However, the level of plasma MDA increased by CCl4 intoxication reduced after treatment (p < 0.05). These results are shown in (Tables 2-4).
Table 2: Effect of Extracts of D. guineense Stem Bark on Markers of Oxidative Stress in Plasma.
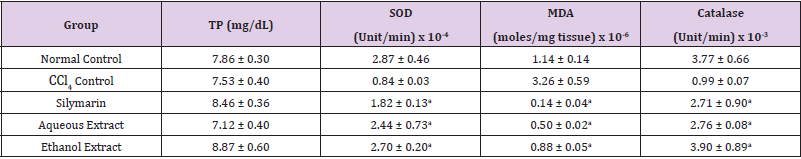
Note: Data are oxidative stress markers and are expressed as mean ± SEM. ap < 0.05, when compared with CCl4 control.
Table 3: Effect of Extracts of D. guineense Stem Bark on Rat Oxidative Status.
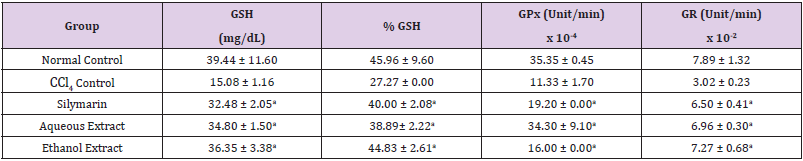
Note: Data are oxidative stress markers and are expressed as mean ± SEM. ap < 0.05, when compared with CCl4 control.
Table 4: Effect of Extracts of D. guineense Stem Bark on NO Level.
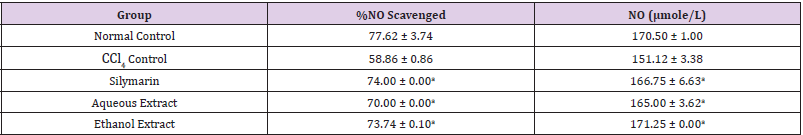
Note: Data are levels of NO and are expressed as mean ± SEM. ap <0.05, when compared with CCl4 control.
Drugs and chemical agents are an important cause of liver injury. Carbon tetrachloride is the most commonly used hepatotoxic agent for the induction of liver injuries in experimental animals. Acute exposure to high levels and chronic inhalation or oral exposure to CCl4 produces liver and kidney damages in humans. It directly impairs organ function by altering the permeability of the plasma, lysosome and mitochondrial membranes. Carbon tetrachloride is metabolized to the noxious trichloromethyl radical (CCl3) by cytochrome p4502E1 (cyp2E1) in hepatocytes [24,25]. The CCl3 causes lipid peroxidation and membrane damage. The radical undergoes anaerobic reactions to form chloroform or carbon monoxide, as well as bind directly to lipid, proteins and DNA [26]. Aerobic organisms possess antioxidant defense systems that deal with Reactive Oxygen Species (ROS) produced as a consequence of aerobic respiration, substrate oxidation or toxicants. Small amounts of ROS, including hydroxyl radical (•OH), superoxide anion (O2•-) and hydrogen peroxide (H2O2) are constantly generated in aerobic organisms in response to both external and internal stimuli [27-29]. The enzymatic and non-enzymatic antioxidant defenses include SOD, GPx, catalase, ascorbic acid (vitamin C), α-tocopherol (vitamin E), glutathione (GSH), β-carotene, and vitamin A [30-32]. For the survival of organisms and maintenance of their health, there is usually a balance between the activities and intracellular levels of these antioxidants [8]. Superoxide dismutase (SOD) detoxifies O2•- which otherwise damage cell membrane and macromolecules [8]. In animals, hydrogen peroxide is detoxified by catalase and GPx. Catalase protects cells from hydrogen peroxide generated within them. It plays an important role in the acquisition of tolerance to oxidative stress in the adaptive response of cells. Catalase prevents drug-induced consumption of O2 [33]. Suppressed action of this enzyme results in enhanced sensitivity of cells to free radical- induced cellular damage [34]. Reduced glutathione (GSH) is a major non-protein thiol in living organism, which act against xenobiotics and neutralize ROS, and disturbances of its intracellular level in biological system has been reported to lead to serious consequences [35]. Malondialdehyde (MDA), a commonly used biomarker of lipid peroxidation, is synthesized from the breakdown of lipid peroxyl radicals during oxidative stress. Measured level of MDA is considered a direct index of oxidative injuries associated with lipid peroxidation [36]. In this study, the activities of all the antioxidant enzymes measured in rat plasma as well as levels of GSH and NO were significantly lower in CCl4 control group than in normal control group, but they were increased by extract treatment. However, the level of MDA increased by CCl4 intoxication reduced after treatment. It is likely that the extracts potentiated the antioxidant system of the rats so as to ameliorate CCl4-induced hepatotoxicity. The plant extract may be used as a potential crude drug for conditions that are caused by oxidative stress. The observed enhanced antioxidant effect may be due to the presence of many important phytochemicals in the extracts of this medicinal plant [15].
This study has provided first-time experimental evidence for the antioxidant properties of extracts of Dialium guineense stem bark in the amelioration of CCl4-induced liver damage.


