Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Wei Zhang Sun1 and Wahib Mahana*1,2
Received: November 29, 2018; Published: December 06, 2018
*Corresponding author: Wahib Mahana Endotoxins, Department of Microbiology, 91405 Orsay, France
DOI: 10.26717/BJSTR.2018.11.002157
Bacterial Lipopolysaccharide [LPS] is Pathogen Associated Molecular Patterns [PAMP] recognized by the Toll Like Receptor 4 complex [TLR4-MD2]. Bordetella genus of Gram- negative bacteria contains nine different species. Of them, Bordetella Pertussis [B.P] infects human and causes the whooping cough, whereas B. Bronchiseptica [B.B], infects different mammals giving respiratory disorders and finally B. Avium [B.A] infects respiratory tract of birds. The aim of this work is to study the specificity and the consequences of LPS interaction with its complex receptors among the different species of Bordetellas and their specific hosts’ cells. Using a specific anti LPS antibody and three cell lines; Human THP1cells, mice Raw cells and chicken HD11 cells, we investigated the binding of the LPS from these Bordetellas to the TLR4 complex whereas LPS from E. coli [E.C] was used as a control. Cells activation was also monitored by measuring the production of three molecules; IL-6, TNF and nitric oxide “NO” in the supernatants of LPS activated cells. Our antibody recognized the three LPS in the ELISA assay. On the cell surface, only the B.P LPS was recognized at the three cell lines followed by B.B then B.A and E.C. Inversely, Cells activation showed that LPS from B.P was the weaker inducer of the three cytokine, whereas B.A that binds weakly to the receptor complex was the best activator of IL-6 production by these cells. Our results indicate the absence of pathogen/host specificity regarding the interaction of LPS with complex receptor.
Abbreviations: BA: Bordetella Avium; BB: Bordetella Bronchiseptica; BP: Bordetella Pertussis; ELISA: Enzyme linked immunosorbent; GAR AP: Goat Anti-Rabbit Immunoglobulin labeled with Alkaline Phosphatase; LPS: Lipopolysaccharides; MD 2: Myeloid differentiation protein 2; PAMP: Pathogen Associated Molecular Patterns; TLR4: Toll-Like Receptor 4; pNPP: p-Nitrophenyl Phosphate.
Lipopolysaccharide [LPS] is the major components of the outer membrane of Gram-negative bacteria. It interacts with Toll-Like Receptor 4 [TLR4] to activate either dependently or independently signaling pathways of the Myeloid Differentiation 88 protein [MYD 88] and initiate the production of pro-inflammatory cytokines [1-3]. Structure of LPS is known to be heterogeneous consisting of three regions and having a variable molecular mass ranging from two to twenty kD [4]. Active component contains variable polysaccharide O specific chain region [O-antigen] specific for the species, a less variable core oligosaccharide of about 10 to 12 sugars, and a relatively conserved lipidic region called lipid A [5, 6]. Bordetella genus has an environmental origin and consists of nine species [7, 8]. Bordetella genus is able to colonize the respiratory tract of human and large number of animals causing variable degrees of respiratory infectious diseases ranging from asymptomatic to sever and chronic diseases [9]. Bordetella Pertussis [B.P] infects strictly human and causes whooping cough [9], B. Bronchiseptica [B.B] infects many mammals including mice and causing kennel cough in the dogs, atrophic rhinitis in the swine and snuffles in the rabbits [10-12], whereas B. Avium [B.A] infects avian causing rhinotracheitis [13]. LPS is variable in composition, biological properties, antigenicity and reactivity among the genus of bordetella [14-17]. Recognition and interaction of LPS with its receptor TLR-4 is crucial to initiate the innate immune response against bortedellosis and other enterobacteriaceae [18]. The interaction of LPS withTLR-4 receptor involves at least three molecules at the cell surface, CD14 binds LPS and transfers it to myeloid differentiation protein 2 [MD-2] which binds to TLR-4 and induces polymerization and signal transduction [19-21]. Additional molecules such as heat shock proteins and chemokine receptors could participate with the complex receptor to induce signaling pathways [22].
At the same time LPS is the major surface molecule and the primary virulence factor of Gram-negative bacteria [4]. A modification of its structure may regulate its various activities allowing the survival and adaptation of bacteria inside the host. To investigate this possibility, we examined the binding of LPS from three Bordetella pathogens [B.P, B.B, and B.A] to the cell surface TLR-4 complex receptor of three cell lines, human THP1, mice Raw and chicken HD 11. We also monitored the LPS activation of the cells by measuring the production of three soluble molecules IL- 6, NO and TNFα, using the LPS from E. coli [E.C] as a control. Our results showed that only the B.P LPS binds correctly to the surface of three cells, followed by B.B then B.A and then E.C LPS. Inversely to the binding, we observed that B. A LPS as well as the E.C LPS are the best inducer of IL-6 and TNFα in both THP1 and Raw cell lines, followed by B.B then B.P LPS. For NO, the results were dependent on the cell line. Our results demonstrate the absence of specificity between the bacterial LPS and the host complex receptor.
Mouse, human, chicken and bovine sera are from GE Healthcare [Velizy, France]. Anti-LPS rabbit polyclonal antibodies were purified from rabbit serum immunized with LPS coupled to KLH using sepharose-protein. A column [Thermo Fisher Scientific- France]. Serum was diluted with PBS then incubated in the column for 15 minutes, washed extensively with PBS and the bound antibodies were eluted using a glycine-HCL buffer [0.1M, PH2.5]. Five mg of purified antibodies were labeled with EZ-Link biotin LC-Hydrazide according to the manufacturer’s recommendation [Thermo Fisher Scientific- France]. Goat anti rabbit immunoglobulin labeled with alkaline phosphatase [GAR-AP] [Clinisciences, Paris]. LPSs from B. Pertussis, B. Bronchisptica, B. Avium and from E. Coli 0119 were purified in our laboratory using lyophilized bacteria. The purity of LPS was checked by SDS-page and silver staining as previously described [8]. LPS was labeled with biotin-LC-hydrazide according as described [23].
Three cell lines were used; The human monocytic cell line, THP1 obtained from Dr. J. Falla- Angel, University of Metz, the mouse monocyte-macrophage cell line Raw 264.7 was purchased from the European Collection of Cell Cultures [ECCAC, Salisbury, UK] and HD11 chicken macrophage cell line was kindly donated by Dr. E. Bottreau, INRA Nouzelly- Tours, France. All cell lines were maintained in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum, 100 IU/ml penicillin and 100mg/ ml streptomycin. Cells were incubated at 37°C in humid air with 5% CO2. To optimize the conditions, fetal bovine serum was mixed either with human serum for THP1cell line, mice serum for Raw cell line and chicken serum for HD11 cell line. TLR-4 knockout splenic cells and normal splenic cells were kindly donated by Dr. E. Garciade- Paco [IBBMC, Orsay].
Cells [2X105/well] were placed in 96 well polystyrene flat bottom plate and incubated with different LPS at the indicated concentrations and time points. The supernatants were then harvested and kept frozen at -200C until use for cytokine titration. Samples were assayed in triplicates and the data were reported as the concentration of tested cytokines. The SEM was less than 10% in all cases.
Indirect ELISA: LPS coated 96 microtiter plates [1mg of LPS per ml in 0.1 M carbonate- bicarbonate buffer [pH 9.6]] were saturated with Phosphate-Buffered Saline [PBS] containing 0.1% Tween 20 and 1% gelatin [PBS-T-G], washed and incubated with anti LPS antibodies at different concentrations for 1 h at 370C . After washing, the plates were incubated with Goat Anti-Rabbit immunoglobulin labeled with Alkaline Phosphatase [GAR-AP] for another h at 370C. The enzyme activity was developed using p-Nitrophenyl Phosphate [pNPP] substrate and the optical density was measured at 405nm on Multiskan EX, ELISA reader [Labsystemes France].
Competitive Test: The 50% fixation point of our anti-LPS antibody bound to the LPS immobilized on the polystyrene plates was determined by the indirect ELISA described above. The antibody was then incubated at this concentration with decreasing concentrations of soluble LPS for 1h at 370C. The mixture was then allowed to react for 1h at 37°C with LPS-coated plates. After washing, antibodies bound to the plates were measured as described above.
Sandwich ELISA: Rabbit anti LPS antibody coated plates [1 mg/ml in carbonate-bicarbonate buffer] were washed, saturated with PBS-T-G, and then incubated with different concentrations of the different LPS in PBS-T-G for 1 h at 370C. After washing, they were incubated with biotinylated rabbit anti-LPS antibody and the reaction was developed as described above, using alkaline phosphatase-conjugated streptavidin [Sigma, France].
Cell surface ELISA: The test was applied according to the method described by Grunow R et al. [24] with few modifications. Microplates 96 wells V bottom were saturated with PBS containing 10% bovine serum and 0.02% sodium azide [200 ml/well]. After washing with PBS, 75 ml of cells suspension [2X105 cells/well] in PBS with 1% bovine serum and 0.2% sodium azide were placed in the well and 75ml of LPS was added and incubated for one h at 37°C. Then the plates were centrifuged at 1500 RPM for 10 minutes and the supernatants were removed by flicking out the fluid phase and tapping slowly the inverted plates on a paper towel to discard the residual fluid. The plates were washed three times the same way using 250 ml/well of PBS. After washing, 100 ml of anti-LPS antibody was added and incubated for one h at 370C followed by second cycle of three washings. After, 100 ml of GAR-AP was added for another h, then washed as above and the reaction was developed by adding 200 ml of pNPP substrate and incubated for 30minute at room temperature. 150 ml of substrate was removed to a new flat-bottomed plate and the absorbance was measured as described above. To confirm the binding of LPS to the cell surface, biotinylated B.P LPS was incubated with the three cell lines for 1h, after washing a solution of alkaline phosphatase-conjugated streptavidin was added for 1h, followed by the substrates. All tests were done in duplicate and the data were reported as the main of optical density or percentage of inhibition of tested antibody. The SEM was less than 10% in all cases.
Cytokine Titration: Tumor necrosis factor-alfa [TNF- a] and interleukin-6 [IL-6] present in culture supernatants of LPS activated cell lines were measured by specific sandwich enzyme-linked immunosorbent assay [ELISA] according to the manufacturer’s instructions [eBioscience, San Diego, CA]. Nitric Oxide [NO] production was estimated by measuring the nitrite concentration of the cell supernatants by the Griess reaction as described in [25]. All samples were tested in triplicate and the data were reported as the concentration of tested cytokines. The SEM was less than 10% in all cases.
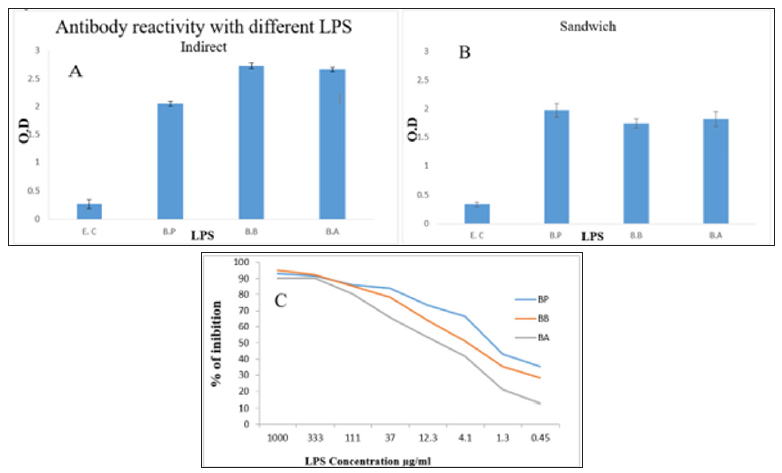
LPS recognition by our antibody was measured by the indirect ELISA assay. LPS from Bordetella genus was recognized in the same order of magnitude as described in Figure 1A. LPS from E. coli, which was used as a control in all tests, had a few reactivity with our antibody. Sandwich ELISA assay showed the same results using the same antibody for capture as shown in Figure 1B. To ascertain the specificity of the reaction, a competitive ELISA test was performed between the soluble and plate fixed LPS. The results confirmed the specificity of our antibody and the 50% of inhibition ranges between 4 and 1mg for the three Bordetella LPS as shown in Figure 1C. For E. coli LPS, the inhibition test was inconclusive due to weak binding.
Figure 1: Antibody reactivity with the different LPS.
A: plates were coated with 1μg/ml of LPS from B.P, B.B, B.A and E.C was incubated with rabbit anti LPS antibody and revealed with goat anti rabbit Ig labeled with alkaline phosphatase using pNPP substrate. B: Rabbit anti-LPS coated plates were incubated with different concentration of LPS and reveled with the biotin labeled anti LPS antibody. The reaction was developed using alkaline phosphatase - conjugated streptavidin. C: Inhibition of rabbit anti LPS antibody by the different LPS. Antibody at 50% binding activity was pre-incubated with different LPS then added to LPS coated plates and the reaction was developed as in A.
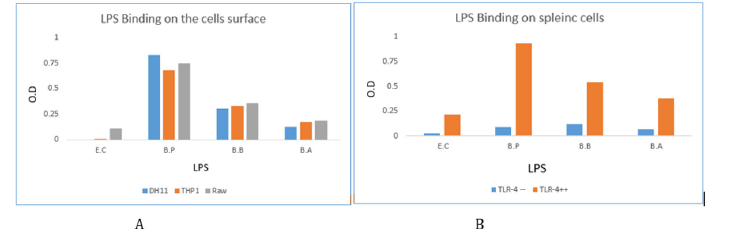
The same antibody was used to visualize the binding of the LPS from different strains of bacteria on the cell surface. To choose the optimal concentration of LPS, a preliminary test using LPS at different concentration [0.01 to 30 mg/ml] were incubated with the cells. After extensive washing the bound LPS was revealed by the antibody. The LPS concentration at 1mg/ml was chosen for further evaluation. Result in Figure 2A showed that LPS from BP88 was the best one for binding to its complex receptor on the cell surface, followed by the LPS from BB. LPS from BA and from control E. coli had a little ability to bind to the cell surface complex. The binding of the LPS to the cell surface was independent from the cell type. BP LPS binds strongly to its complex receptor on the three tested cell lines. To verify the specificity of the interaction of LPS with its TLR4 complex receptor, LPS was tested for its binding on the cell surface of splenic cells from TLR4 knockout mouse and from normal mouse. The result in Figure 2B showed no binding on the cells from knockout mouse. In addition, the role of the serum elements was tested by mixing fetal bovine serum with human AB serum for THP1 cell line, mice serum for Raw cell line and chicken serum for DH11 cell line. No significant differences were observed for each cell line [data not shown].
Figure 2: LPS binding on the cell surface.
A: LPS from different Bordetella and E.C at 1g/ml was incubated with the different cell lines, then reacted with rabbit anti LPS antibody and goat anti rabbit Ig labelled with alkaline phosphatase and the reaction was developed using pNPP substrate. B: LPS binding on splenic cells from TLR4 knockout (TLR--) and TLR4 positive cells (TLR++). Cells were incubated with LPS for one h and incubated with rabbit anti-LPS antibody and goat anti-rabbit Ig labelled with alkaline phosphatase.
The activities of LPS on cytokine production depend on the origin of the LPS and time of incubation. To determine the appropriate concentration and the time of incubation, a preliminary test was performed. The three cell lines were incubated with different concentrations of LPS and for different incubation times; 6h, 12h, 24h, 48h, 72h, 138h, 144h and one week. After this, the supernatants were removed for measuring the levels of cytokines and for assessing the viability of the cells. According to these preliminary results, we used different concentrations of LPS: 10 ng for E.C and 80 ng for Bordetella LPS of B.P, B.B and B.A. and the Time of incubation was adjusted to 24 h and the levels of these molecules TNFα IL-6 and NO were measured.
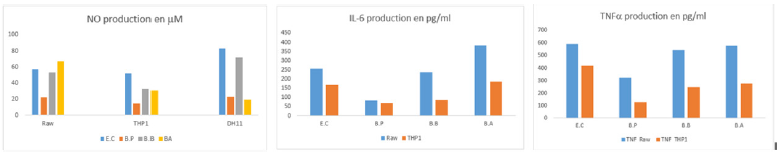
In addition, the role of the serum used in these tests was evaluated. The results of the cytokines production showed no significant differences either in presence of absence of the specific sera. The major finding indicates a slight but not significant amelioration in the Raw cells activated with B.A which showed the highest augmentation of the NO production in the presence of mouse serum as demonstrated in Figure 3. Results in Figure 3A showed the production of Nitric Oxide [NO] by the three cell lines. For Raw cell line, we found that BA, EC and BB LPS produced comparable amounts of NO ranging from 2.5 to 3 times more than the amount produced by BP. The results for THP1 cell line showed significant difference between E. coli LPS and LPS from BB and BA, which produced two times more NO than BP. However, HD11 cell lines showed differential patterns E.C and B.B LPS produced four times more NO than B.P and B.A LPS. For IL-6 production, we found that Raw cell line produced more IL-6 than THP1 cell line in response to all LPS tested, and that BA LPS was the best inducer of the IL-6 followed by E.C and BB LPS. In contrast, B.P LPS had a very week activity in inducing IL-6 production by the two cell lines Figure 4B. THP1 cells produce lower amounts of IL-6 in response of all LPS, whereas B.A and E.C LPS induced similar production, followed by BB. The results of TNFα shown in Figure 4C confirmed the fact that Raw cell line responds better than THP1 cell line. E.C, B.B and B.A LPS produced comparable amounts of TNFα, which was significantly higher than the amount produced by the BP LPS. For THP1, we observed that E.C LPS was the best inducer of TNFα by this cell line, BA and BB had relatively the same production of TNFα, and BP LPS induced low TNFα production.
Figure 3: Role of specific serum on the production of NO by stimulated cells. Cells were incubated with different LPS at the concentration of 10 ng for E.C LPS and 80 ng for Bordetella LPS in presence (bleu) or absence (orange) of 5% mouse serum for Raw cell line or 5% chicken serum for DH11 cell line for 48 h. The concentration of NO was measured in the supernatants as described in materials and methods.
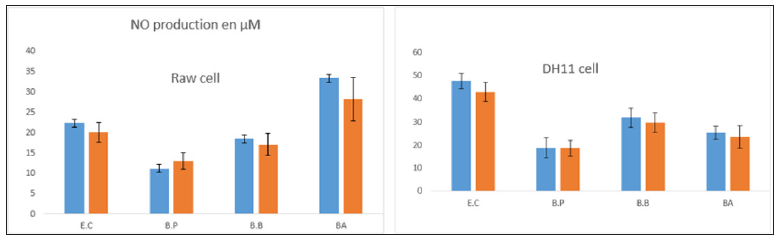
Figure 4: Cytokines production by activated cells. Raw, THP1 and DH11cell lines were incubated with different LPS at 10 ng concentration of for E.C LPS or 80 ng for Bordetella LPS during 24 h for TNF and 48 h for IL-6 an NO measurement. After the indicated times the supernatants of activated cells were harvested and the concentration of cytokines was determined as described in materials and methods.
In this report, we presented for the first-time results indicating the absence of specificity between the pathogen and its host regarding the interaction of LPS with the TLR4-MD2 complex receptor. Using LPS from three Bordetellas; B. P, the agent of wooping caugh, B. B, the agent of many infections in mice and other mammals, B. A, agent of respiratory infections in birds and as a control the LPS from E. coli. The LPS from the three Bordetella were recognized strongly by our antibody in the different ELISA tests; Direct, sandwich and competitive, which confirms the specificity of this antibody. In contrast, this antibody recognized differently the LPS on the cell surface of the three cell lines isolated from human, mice and chicken. It is plausible that this recognition may be due to the amount or the orientation of LPS fixed on the cell surface. The specificity of pathogen/host interaction was studied at two levels in this report. First is the binding of LPS to the TLR4 complex receptor and the second is the ability to induce the production of three soluble molecules. The specificity of the binding of LPS to TLR4-MD2 complex receptor on the cell surface was supported by the absence of the signal using the spleen cells from TLR4 knockout mice.
The Lipid A moiety is the biologically active part of LPS molecule able to activate the innate immune response [5,6,26]. It is not exactly known how the recognition of LPS is by the TLR4- MD2 complex in birds. However, in human and mouse the E. coli lipid A is recognized in similar way by both complex receptor TLR4-MD2 [27,28]. Crystallography studies looking to understand the interaction between LPS and its TLR4-MD2 complex receptor was done using purified molecules. In our study, we used purified LPS and antibody but on the other side, we used the TLR4-MD2 complex receptor present in its natural form on the cell surface. In this situation, the test may be closer to the physiological condition and may reflect the actual complications due the involvement of different interacting factors at the cell surface. The crystal structural analysis of the LPS recognition by the TLR4-MD-2, in both human and mouse showed that the binding of LPS induces the formation of a dimer of two copies of the TLR4-MD-2-LPS [27,28]. These studies and others [29,30] showed the importance of the number of fatty acid acylation in the lipid A as well as hydrophobic and uncharged amino acids in TLR4-MD2. In our work, the number of acylated fatty acid on the LPS alone could not explain the difference of the binding to the cell complex receptor because the best binding was obtained with BP LPS, which has penta-acylated LPS and the other three LPS are hexa-acylated. However, we could not determine the binding of the E. coli LPS, which is hexa-acylated. On the other hand, the ability of different LPS to bind to the TLR4-MD2 complex receptor at the cell surface and the ability to induce the production of proinflammatory cytokines do not follow the same order. For example, LPS from BP, which had the best binding to the complex receptor is the lowest inducer of the cytokines by the three cell lines. In contrast, LPS from BA which binds weakly to the complex receptor was able to better induce the production of the cytokines. The ability to bind the complex receptor was independent from its biological activity at least in our system. These results indicate the presence of other factors influencing the binding of LPS to TLR4- MD2 complex receptor and that its biological activity and lipid A alone could not be independent of two other part of the LPS.
Cytokine production after infection plays an important role in the immune response against the bacteria and decreased cytokine production due inefficient TLR4 stimulation enables bacteria to avoid host immunity [31]. Different previous studies showed the variation of LPS activity related to the structural change or to the host conditions. It has been reported that substituting the BP lipid A phosphate group with glucosamine increases the release of proinflammtory cytokines in cells expressing human but not murine TLR4-MD2-CD14 [32]. Different activity of LPS from BP and Bordetella parapertussis on human dendritic cell functions was reported and related to the CD14 dependence, cytokines production and signaling pathways activation [33]. Temperature dependent shift in the lipid A of Yersinia pestis LPS when grown at 37°C that differentially affects recognition by mouse versus human TLR4/MD2. LPS produced at this temperature is hypo-acylated and less stimulatory to human compared with murine TLR4/MD2 [34]. Moreover, pro-proliferation and anti-proliferation opposing effect of LPS on murine leukemia cell was reported which could be due to the increased production of IL12 [35].
The adaptation between bacteria and their hosts is a long process established during evolution, in which some specific modifications tack place in both partners. The mechanisms of such specificity are not clear, but in some cases, specificity was determined by surface receptors that allow penetration of the pathogen or their virulence factors into host tissues [36]. In our study, the three pathogens are adapted to their host i.e. BP in human, BB in mice and BA in birds. However, the interaction LPS with its complex receptor TLR4-MD2 is not specific. The three forms of LPS bind the same way to the receptor complex on the three cells belonging to the different species, but differentially induced the production of the proinflmamtory molecules. In conclusion, our study indicates the absence of Bordetella LPS specificity for its complex receptor on the cells from the hosts and the absence of the relation between the strength of the binding to the receptor complex when correlated to its biological activity.
We thank the international office for exchange program of our university, the Agence University of the Francophonie [AUF] and the College doctoral Franco-Chinois for the financial support of Wei Zhang-Sun. We thank also E. Bottreau. E. Garcia-de-Paco and J. Falla-Angel for providing us with the cells..


