Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yan Tu1, Yazhuo Shang*1, Honglai Liu1 and Fei Gao2
Received: August 01, 2018; Published: August 08, 2018
*Corresponding author: Yazhuo Shang, School of Chemistry & Molecular Engineering, East China University of Science and Technology, China
DOI: 10.26717/BJSTR.2018.07.001542
The mixed systems of bovine serum albumin (BSA) and surfactants were widely used in biological, foods, pharmaceutical and cosmetics. In this work, trimeric (methyldodecylbis [2-(dimethyldodecylammonio) ethyl] ammonium tribromide surfactant (C12-C2-C12-C2-C12·3Br-) and Gemini surfactant 1, 2-ethane bis (dimethyldodecylammonium bromide) (C12-C2-C12·2Br-) were synthesized. And the interaction of trimeric surfactant (C12-C2-C12-C2-C12·3Br-), Gemini surfactant (C12-C
Keywords: Bovine Serum Albumin; Trimeric Surfactant; Gemini surfactant; Interaction
Abbreviations: DTAB: Dodecyl Trimethyl Ammonium Bromide; DLS: Dynamic Light Scattering; CMC: Critical Micelle Concentration; CAC: Critical Aggregation Concentration; BSA: bovine serum albumin
Protein is a kind of important biological organisms [1]. Due to the amphiphilic properties of the amino acids, which inducing the binding of surfactants on protein [2]. It is well known that the surfactants-protein system are of significant importance because of their widely used in cosmetic, biological, pharmaceutical, drug delivery systems [3-6]. The surfactants can be used as denaturants to organize the structure of protein which in many cases leads to perturbation of various biological functions of protein [7]. For instance, cationic surfactants can be used as bactericide in various protein systems [8]and Alzheimer’s disease due to aggregation of amyloid genic proteins [9]. Therefore, understanding of the nature of protein-surfactant interaction seemed to be essential with respect to the advancement of the fields of pharmaceutical and cosmetic research [10,11]. Up to now, the interaction of protein with surfactants has been widely reported. Previous investigations on BSA/surfactant were mainly focused on the traditional surfactants, Such as DTAB, SDS, and C12E8 [12-14]. Later, the research showed that Gemini surfactants made a lager effect on proteins as compared with conventional single-chain surfactants [14-18]. Then a large amount of literatures about Gemini surfactant / BSA system were appeared [19-21].
Liu’s group [22] investigated the interaction of Gemini surfactants (12-n-12, n=3, 4, 6) and made a comparison with the traditional single-chain surfactants (DTAB). The results showed that the interaction between BSA and Gemini surfactants increase with the length of spacer groups and the BSA/Gemini surfactants systems is superior to BSA/DTAB system. Up to now, the research of surfactant/BSA system still mainly about Gemini surfactant. However, the oligomeric surfactants have rapidly develop. Gemini surfactant which has many unique properties [23,24] drive people to research the higher oligomeric surfactants, such as trimeric surfactants. However, no relative research about the BSA/ trimeric surfactant complex has been published. Therefore, it’s meaningful for us to research the interaction of BSA with trimeric surfactants. Which may broaden the application of novel trimeric surfactants in industry area. In this article, we investigated the interaction of trimeric surfactants with BSA and make a comparison systematically with Gemini surfactant and monomeric surfactants. BSA is widely used as a model protein for its structural homology with HSA, low cost, high water solubility, easily available in pure form [25].
It is a kind of globular protein with a relative molecular mass of around 66KD. The primary structure of BSA is composed of 583 amino acid residues and it is characterized by low tryptophan content along with a high content of cysteine, stabilizing a series of nine loops. The tertiary structure is composed of three domains I, II, and III, with each containing two subdomains A and B. The protein BSA contains two tryptophan residues, Trp-134 and Trp-212, and they are located in the hydrophilic subdomain IB and hydrophobic subdomain IIA, respectively [26]. The cationic quaternary ammonium trimeric surfactants which have three hydrophilic head groups and three hydrophobic chains linked by spacer groups were synthesized and characterized. We investigated their interaction with BSA by surface tension, fluorescence methods dynamic light scattering (DLS). Cationic quaternary ammonium trimeric surfactants with hypo toxicity, lower critical micelle concentration, and higher interfacial activity and so on compared with Gemini surfactants, which made it as an alternative to be used in biochemistry. Furthermore, the trimeric surfactants interact strongly with protein molecules and denature them at very low concentrations.
Thus the trimeric surfactants could behave as an efficient denaturant than the common chemical denaturants such as urea guanidine hydrochloride etc. and in such altered conformational states, protein molecules can readily interact with nonpolar substrates [27]. The further study of interaction of BSA/surfactants is of great significance to deeply understand the interaction principle of surfactant with BSA and promote the development of medicine and food engineering. It can predict that trimeric surfactants has better properties compared with corresponding Gemini surfactants in surfactant/proteins systems. It will also enrich the application of surfactants in the field of biology.
Materials
The Trimeric (Methyldodecylbis [2-(Dimethyldodecylammonio) Ethyl] Ammonium Tribromide (C12-C2-C12-C2-C12·3Br-) was synthesized according to the methods reported elsewhere [28]. The Gemini surfactant 1, 2-ethane bis (dimethyldodecylammonium bromide) (C12-C2-C12·2Br-) was synthesized according the paper we have published [29]. DTAB and Bovine serum albumin (BSA, fraction V) was purchased from Aladdin (Shanghai, China). The structure of BSA, C12-C2-C12-C2-C12·3Br-, C12-C2-C12·2Br- and DTAB are shown in scheme1. The ultrapure water was used to prepare the sodium phosphate buffer of PH=7.4 with ionic strength being 0.02 mol·L-1 by Na2HPO4 and NaH2PO4 (Sinopharm Chemical Reagent Co., Ltd). All solutions were prepared using sodium phosphate buffer solution as solvent
Structure and property characterization
The fluorescence measurements were obtained by using Hitachi F-4500 fluorescence spectrometer. The sample was measured using a 1cm quartz cuvette at 298.15K, 308.15K and 318.15K. The slits of excitation and emission were both fixed at 5.0nm and 2.5nm respectively in fluorescence measurement. The intrinsic fluorescence of BSA solutions (1g/L) with various surfactant concentrations was measured by fixing the excitation spectra at 280nm and the emission spectra ranging from 290 nm to 400 nm.
Dynamic light scattering (DLS)
DLS measurements of BSA with different concentrated surfactants were taken by Malvern Nano ZS instrument (Southborough, MA) at 298.15k with a 173° backscattering detector and a He-Ne laser of 633nm. All samples were filtered in a quartz cuvette about 1ml by 0.22um microporous filters and keep constant temperature 120s before measurement. The hydrodynamic radii was obtained using the DLS software.
The surface tension of C12-C2-C12-C2-C12·3Br- and C12-C2-C12-C2-C12·3Br-/BSA
The CMC of C12-C2 -C12-C2-C12·3Brmeasuredby surface tension method are 0.82×10-4 mol/L. The CMC of trimeric surfactants is an order of magnitude lower than the corresponding Gemini surfactant C12-C12-C12 ·2Br-. Surface tension is an effective method to study the interaction between protein and surfactants. From figure, it can be seen that the surface tension of complex is lower than the surfactants. Which indicate that the complex is more surface active compared to surfactants. The first break point of the BSA/surfactant system is the critical aggregation concentration (CAC). After CAC, a more or less constant surface tension region is observed until the protein backbone is saturated by surfactants. The break point is called protein saturation concentration (Cs). After the CS, The surface activity starts to decrease again with the further addition of surfactants and then reach the CMC. The CMC of complex is larger than corresponding pure surfactants due to the binding of surfactants to protein and more surfactants required to saturate surface. The results show that there is a significant interaction between BSA and trimeric surfactant.
Intrinsic fluorescence
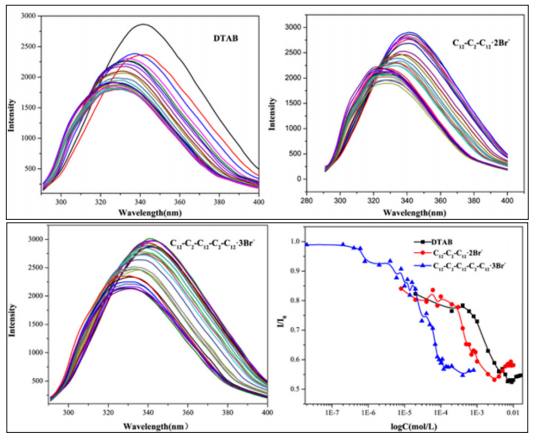
The structure of BSA is sensitive to fluorescence due to tryptophan (Trp) and tyrosine (Tyr) residues. Thus, fluorescence investigations is an effective way to evaluate the interaction of BSA with surfactants [8]. As a result, the interaction of three surfactants with different aggregates with BSA was compared by intrinsic fluorescence method. As shown in (Figures 1 & 2), the fluorescence intensity does not change significantly at lower surfactants’ concentration. With the further increase of the concentration of surfactants, the values of fluorescence intensity reduce drastically. It suggests that the BSA is unfolded gradually while the hydrophobic group (tryptophan) of BSA is exposed to the hydrophilic environment. Fluorescence measurements indicate that the interaction is expected to be mainly electrostatic at lower surfactant concentrations, and when the surfactant concentration increases, hydrophobic interaction plays a major role. Further increase surfactant concentrations, BSA−surfactant interactions are saturated and a subsequent increase in surfactant concentration results in the formation of micelles. Besides, the concentration of trimeric surfactant C12-C2 -C12-C2-C12·3Br-causing BSA conformational changes is much lower than the concentration of Gemini surfactants (12-C2-C12·2Br-) and monomeric surfactants (DTAB). In addition, we can also see from the comparison of fluorescence curves of three surfactants that when the concentration of surfactants are close to CMC, the conformation of protein can be changed to maximum. As you can see from the figure, the order isC12-C2 -C12-C2-C12·3Br-> C12-C2 -C12-C2-C12·3Br->DTAB. The results showed that the trimeric surfactants made a stronger effect on BSA
Figure 1: Surface tension curves of C12-C2-C12-C2-C12·3Br- and C12-C2-C12-C2-C12·3Br-/BSA (1g/L) at PH=7.4, T=298.15k.
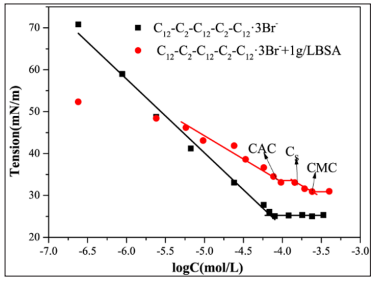
Figure 2: Intrinsic fluorescence of BSA(C=1g/L) with various surfactants (DTAB; C12-C2-C12·2Br-; CC12-C2-C12-C2-C12·3Br-) at PH=7.4 and 289.15K
Dynamic light scattering (DLS)
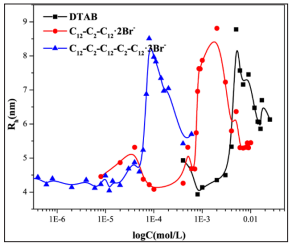
Studies have shown that the hydrodynamic diameter of the protein would increase when the surfactants were added into the protein aqueous solution [30]. Due to the hydrophobic interaction between the hydrophobic tail chains of surfactants and the non-polar amino acids on the protein. The hydrophobic amino acids hidden in the protein core can be unfolded, resulting to the denaturation of protein. Therefore, the effect of different surfactants on BSA conformation can be compared by the variation of hydrodynamic radius of BSA/surfactant complexes. It can be seen from the (Figure 3) that the hydrodynamic radius (Rh) of the protein changes little at the lower concentration of surfactants. With the further increase of the concentration of surfactants, the hydrophobic interaction between the surfactants and BSA leads to the unfolding of BSA. So that the Rh increases. Besides, the electrostatic interaction between the surfactants and BSA reduce the electrostatic repulsion among BSA molecules. It also leads to an increase of the Rh of samples. As the surfactant concentration is further increased, the surfactant micelles are gradually formed, so that the hydrodynamic radius decrease. DLS studies reveal that the hydrodynamic diameter of BSA increases about 2 times when the protein-surfactant assemblies are formed. It verified that the electrostatic interaction pays an important role of in controlling the overall formation of the BSA-surfactant assembly. It can be seen from the (Figure 3) that the concentration of the C12-C2-C12-C2-C12·3Br- to denature the BSA is much lower than the corresponding Gemini surfactant C12-C2-C12·2Br- and single chain surfactant DTAB, indicating that the trimeric surfactant is much more denatured than the traditional surfactant. This is consistent with the results obtained by intrinsic fluorescence
Figure 3: The variation of hydrodynamic radius of BSA/surfactant complex with concentration of different surfactants at PH=7.4 and 289.15K.
The comparative studies on the interactions of bovine serum albumin (BSA) with cationic trimeric surfactant C12-C2-C12-C2-C12 ·3Br-, Gemini surfactant C12-C2-C12·2Br- and single-chain surfactant DTAB have been carried out. Fluorescence measurements indicate that the hydrophobic interaction plays a major role in unfolding the BSA verified that the electrostatic interaction pays an important role of in controlling the overall formation of the BSAsurfactant assembly. Those results show that there is a cooperation of electrostatic interaction and hydrophobic interaction for surfactants/BSA system in controlling the protein formation. For both the electrostatic interaction and hydrophobic interaction, the trimeric surfactants are expected to be stronger than corresponding Gemini surfactants and single-chain surfactants, the former are more efficient as protein denaturants than the latter. The results show that the aggregation degree of surfactant has a significant effect on the conformational changes of BSA. It is suggested that the synthesized trimeric surfactants can prove as effective tools for the refolding of proteins. In future, these findings can be used as a model to study other such systems and broaden their application in the fields of pharmaceuticals, cosmetics, biochemistry.
This work is supported by the National Natural Science Foundation of China (Project No. 21476072) and the Fundamental Research Funds for the Central Universities


