Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
*Corresponding author:
Tadeusz Michałowski, Department of Analytical Chemistry, Technical University of Cracow, Cracow, PolandReceived: July 18, 2018; Published: August 16, 2018
DOI: 10.26717/BJSTR.2018.08.001591
To view the Full Article Peer-reviewed Article PDF
The linear combination f12 = 2⋅f(O) – f(H) of elemental balances: f1 = f(H) for H and f2 = f(O) for O is the keystone in formulation of Generalized Electron Balance (GEB), which completes the set of equations necessary for resolution of an electrolytic redox system according to Approach II to GEB in aqueous media. The f12 does not involve the numbers of free and hydrating water molecules. The linear dependency or independency of f12 from charge balance (f0 = ChB) and other elemental/core balances fk = f(Yk) (k=3…,K) for elements/cores Yk of the system is the general criterion distinguishing between non-redox and redox systems. This criterion is provided by linear combination 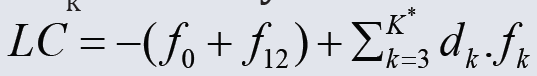 , where K* (≤K) is the number of electron-non-active elements (fans) of the system, and dk are the multipliers related to oxidation numbers (ONs) of the elements involved in Yk. For non-redox systems, LC is transformed into identity, 0 = 0, whereas for redox systems, the LC and each linear combination
, where K* (≤K) is the number of electron-non-active elements (fans) of the system, and dk are the multipliers related to oxidation numbers (ONs) of the elements involved in Yk. For non-redox systems, LC is transformed into identity, 0 = 0, whereas for redox systems, the LC and each linear combination  , where ak ∈ ℝ, are different from identity. The ONs, reductant, oxidant are derivative concepts in this formulation. All these interesting regularities are illustrated by simulated titration in a symproportionating redox system, resolved according to Generalized Approach to Electrolytic Systems (GATES) principles.
, where ak ∈ ℝ, are different from identity. The ONs, reductant, oxidant are derivative concepts in this formulation. All these interesting regularities are illustrated by simulated titration in a symproportionating redox system, resolved according to Generalized Approach to Electrolytic Systems (GATES) principles.
Abstract | Introduction | Balancing of Electrolytic Systems – General Remarks | An example of D+T System | Final Comments | References |


