Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
José Refugio Parga Torres1*, Esteban Sánchez Valdés1, Francisco Cepeda Rodríguez2 and Víctor Vázquez Vázquez3
Received: December 19, 2025; Published: January 09, 2026
*Corresponding author: José Refugio Parga Torres and Esteban Sánchez Valdés, department of Metallurgy and Materials Science, Tecnologico Nacional de Mexico – Instituto Tecnologico de Saltillo. Blvd V. Carranza 2400, Col. Tecnológico, Saltillo, Coahuila. C.P.25280, México
DOI: 10.26717/BJSTR.2026.64.010039
The recovery of precious metals by cementation reactions are used extensively in extractive metallurgy processes where the removal of more noble ions from cyanide solutions is desired. Even through the reactions may be written in very simple terms, the actual process of the electrochemical reduction cementation is often found to be very complex. Recovery by Electrocoagulation technology for precious metals and heavy toxic metals have progressed in line with new novel techniques in this century, while the classical treatment Merrill Crowe zinc dust electrochemical process remains essential for profitable production of gold and silver production. Actually, the EC process provide excellent results when applied to very low concentrations cyanide ions of precious metals and also when the rich cyanide solution contains complex cyanide ions of copper. In this research basic studies have been conducted on the kinetic cementation of gold / silver the results showing the reaction to be the first order reaction and diffusion controlled. In this research X-ray Diffraction, Scanning Electronic Microscope and Atomic Adsorption were used to characterize the solid products formed during the EC process. The results suggest that magnetite particles and amorphous iron oxyhydroxides are present (Lepidocrocite and Gohetite). The EC products recover gold and silver from cyanide solutions within 10 minutes and recoveries of gold and silver 98 % and simultaneously in a hermetic reactor the recovery of sodium cyanide was 95%. Finally, the Dore product has 96% pure and this a precursor to make superconductors.
Keywords: Sulphide Precipitation; Merrill Crowe; Electrocoagulation; Dore
Gold cyanidation process has been reported to involve the chemical reactions shown in Equations (1) and (2). Silver is accomplished in the same fashion reactions:
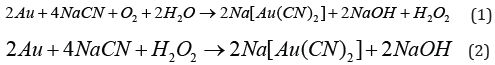
The equation proposed by Elsner 150 years ago, is stoichiometrically correct but does not describe the cathodic reactions associated with the dissolution. Details of this electrochemical reaction have received considerable attention and under certain circumstances the reaction is limited by the coupled diffusion of CN- and O2 to the gold surface. Therefore, the stoichiometry of the process shows that 4 moles of cyanide are needed for each mole of oxygen present in solution. At room temperature and standard atmospheric pressure, approximately 8.2 mg of oxygen are present in one liter of water. This corresponds to 0.27 x 10-3 mol/L. Accordingly, the sodium cyanide concentration (molecular weight of NaCN = 49) should be equal to 4 x 0.27 x 10-3 x 49 = 0.05 g /L or approximately 0.01%. This was confirmed in practice at room temperature by a very dilute solution of NaCN of 0.01% - 0.5% for ores that contain gold /silver, and for concentrates rich in gold and silver of 0.5% -5% [1]. Details of this electrochemical reaction have received considerable attention and under certain circumstances the reaction is limited by the coupled diffusion of CN- and O2 to the cathodic gold surface. Lime or sodium hydroxide (caustic) is added to keep the system at an alkaline pH of 10-11. This protective alkalinity is required to counteract the generation of acid during cyanidation, thereby preventing cyanide degradation and the formation of the deadly HCN gas. The dissolution of gold / silver in dilute solution of cyanide ions in alkaline medium with air is a very slow process (48 – 72 hours) [2,3]. However, with the use of pressure oxidation leaching and temperature revels stubble surface changes the appearance porous or pits at the surfaces of crystals of Argentopyryte (AgFeS3) or pyrite (FeS2), Figure 1 show this behavior to efficient the leaching pression.
By using pressure and temperature reaction in the operation reactor the leaching of dissolve gold and silver was in 90 minutes. Also, this phenomenon of dissolution its accelerated by the presence of ferric ions from the oxidation of the pyrites ores with the oxygen equations 3 to 5.
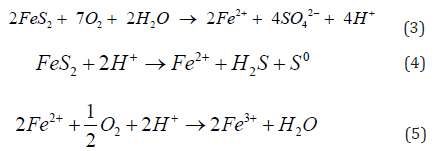
The ferric ions also contribute to the oxidation of arsenopyrite, argentite, pyrite, pyrrhotite, sphalerite, and chalcopyrite see equations 6 to 11:
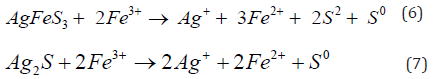
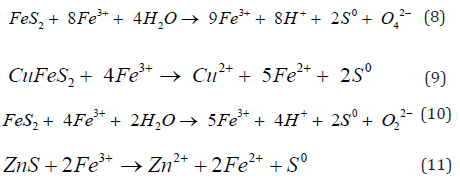
Of course, silver has been shown to activate the oxidation and dissociation of chalcopyrite because of porous sulfur layer is formed [1]. Then, elemental sulfur may also be further oxidized to sulfate by oxygen or by ferric sulfate:

This results in the formation of a porous, but nonprotective, elemental sulfur layer, thus allowing cyanide and dissolved oxygen to access to the previously locked gold and silver. This can be represented in Figure 2, which shows an example of this behavior in a porous sample with a high gold content (0.98 weight %).
Leaching Metals Complex Cyanide with Ions Cyanide
The concentration of cyanide used to dissolve gold in ores is typically much higher than the stoichiometric (eq. 14) amount required, owing to the solubility of other minerals. Free cyanide produces complexes with several metallic species, especially transition metals, which show a broad variation in both stability and solubility [4-6]:
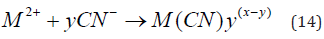
Many common zinc and copper minerals are soluble in the dilute cyanide solutions typical of leach conditions found in gold cyanidation processes [7,8]. Then, the next process for recovery of gold and silver from the pregnant cyanide solutions is the zinc dust Merrill Crowe process the increasingly stringent limitations on the purity of the Dore for the refining process have created a growing interest in the development of a novel process for the removal of copper, Zinc, lead, and Cadmium cyanide ions from the pregnant solution before cementation in the filter press. Figure 3 show the Merrill Crowe process indicating its stages and the time in cyanidation, filter press and refining to obtain Dore with 96% purity.
Laboratory Autoclave Leaching Cyanidation / Oxidation Experiments
First, the concentrate is regrinded in a ball mill for several hours to achieve an average particle diameter in the range of 74–20 microns. Then, cyanide and lime are added to this concentrate, which is then placed in an autoclave with varying pressure and temperature, the autoclave reactor is shown in Figure 4. The capacity of the Parr Pressure reactor Vessels is 1-gallon (3.75 liter) size. These vessels are available with an FKM O-ring seal for operating temperatures to 30-225°C, and FFKM O-ring for temperatures to 250°C, or with a flat, PTFE gasket for operating temperatures up to 250°C maximum. And the operating conditions for this equipment are shown below in the Table 1. By using these optimal conditions in the laboratory pressure leaching reactor the results show that particle less 20 microns give recovery of gold 96 % and silver 94, see Table 2. A graph of these results is shown in Figure 5, where to obtain good gold and silver recoveries in the autoclave it is necessary to regrind the concentrate to an average particle diameter of less than 20 microns. According to these results, the mill that can provide this parameter of 10 microns is the ISAMILL manufactured by the Australians. Previous results showed that the dissolution of gold (logβ2= 39.3) was better than that of silver (logβ2= 20.48) because the stability constant of gold is greater. Electrolytic Process for Recovering Gold and Silver from a Cyanidation-Rich Solution by Electrocoagulation Technology. After the simultaneously pressure oxidative cyanidation leaching of the concentrate, 8 liters of rich solution were obtained by using a pressure reactor, the chemical analysis of the pregnant solution is shown below in Table 3. This gold- and silver-rich cyanide solution will be used to confirm the feasibility of using electrocoagulation process for precious metal recovery. For this electrochemical process, which involves gold and silver cyanide ions, an electrolytic cell with iron and aluminum anodes and cathodes was used. The preliminary results, shown below, are acceptable for both metals; however, the iron electrodes yielded the best results, with a treatment time of five minutes and a recovery rate of 98%. Treatment of wastewater by EC has been practiced for almost a century with limited popularity, this technology has become increasingly popular around the world for treatment of industrial wastewater containing metals [9-12]. Electrocoagulation is an innovation processes that offer significant potential for removing soluble ionic gold and silver from cyanide rich solution. EC operating conditions highly dependent on the chemistry of the aqueous medium, especially conductivity and pH. Other influent characteristics such as particle size, type of electrodes, retention time between plates, plate spacing and chemical constituent concentrations dictate the operating parameters of this electrochemical process. Typically, empirical studies are done on EC to define major operating parameters for recovery gold and silver [13-15]. Figure 6 shows the reaction mechanism at the anode and cathode for the recovery of Au and Ag metals.
Laboratory Electrocoagulation Experiments
The electrocoagulation apparatus consisted of a flow-through cell, the electrode assembly, the feed pump and the power supply. A picture of the electrocoagulation reactor is shown in Figure 7. The EC reactor contained five parallel carbon steel electrode plates (11.0 cm x 11.4 cm) spaced with 6mm between each forming four parallel cells. The internal volume of the cell is approximately 1200 ml and a variable transformer was used to control the current and potential. Analysis of Au and Ag were conducted to the Basis solution, by AES. EC was run at 15 Volts (DC) and the corresponding current was of 0.1 A. EC was run for five minutes, and a sample was taken every minute in order to determinate the removal efficiency for Au and Ag. The chemical analysis of the pregnant cyanide solution is shown on the Table 4 form together with conditions of EC experiment with variations of time. In Figure 8 is shown EC results and the recoveries for gold and silver are bigger than 96% of the recovery for both elements. In Figure 8 is shown EC results and the recoveries for gold and silver are bigger than 96% of the recovery for both elements. In the Figure 9 is shown X-ray diffraction analysis of solids obtained in the EC recovery of gold and silver in cyanide C: Cu2Fe(CN)62H2O, Ag: Silver, M: Magnetite and L: lepidocrocite species. In Figure 10, illustrates the results of the EDAX of the sludge from the EC process, in this electrochemical technology the gas bubbles being produced by electrolysis are expected to carry the gold and silver to the top of the aqueous solution where it is concentrated, collected and removed with the magnetic species of iron. The gold and silver cyanide ions react with the OHions produced at the cathode during the evolution of hydrogen, to yield insoluble hydroxides that will adsorb gold and silver from the leach solution from the Pachuca tank and also contribute to coagulation by neutralizing the negatively charge colloid particles that may be present at neutral or alkaline pHs. This enables the particles to come together closely enough to agglomerate under the influence of van der Walls attractive forces.
Complex Cyanide and Sodium Cyanide Recovery Process
Table 5 shows solubility of metal hydroxides and sulfides. Both methods involve a reaction of the metal cation with either OH- or S to form the corresponding insoluble metal hydroxide or sulfide, as shown below:

In metal finishing wastewater, multiple metals are present at high levels. Therefore, the most effective pH must be determined prior to treatment (see Table 5). For most metals, the sulfide precipitate has a much lower solubility in aqueous solutions than do their hydroxide counterparts. This makes sulfide precipitation an attractive process when dealing with very low emission limits. In this research the sulfide precipitation offers great advantage for ions in cyanide solution. Although some metals can be selectively precipitated by the sulfide precipitation treatment method by adjusting the pH, selective metal precipitation is generally not possible. Based on differences in metal sulfide solubility’s, copper can be effectively separated from nickel, zinc, lead, and cadmium. In all the tests the barren initial solution from the Merrill-Crowe Minera Williams plant was from the same batch (0.1 Ag ppm, 280 Zn ppm, 2500 Cu ppm, 7 Fe ppm). The following conditions were also fixed: temperature 250C; stirring speed 200 rpm; 0.5 to 2 g/l Na2S and reaction time 90 seconds. The experimental results of the copper, silver, zinc and iron precipitation at different pH are presented in Table 6. The results showed that pH had a great effect on copper cyanide removal efficiency and the optimum pH was about 3 to 4.0. At this pH value copper cyanide removal efficiency could be achieved above 99 %, when influent copper concentration ions were 636 ppm. Some black precipitates were observed in the solution samples of the pH 2 to 6 experiments, which suggested that there was copper, silver, arsenic, zinc and iron as sulphide. The production of this sulphide is confirmed by X-ray diffraction, as shown in Figure 11. The measured sample, which was collected from experiment pH = 3 (see Table 6), gives rise to peaks corresponding to covellite, chalcocite, argentite and esfalerite. The size, EDAX and morphology of the solids in experiment pH are also shown in Figure 12 by SEM and EDAX analysis.
Industrial Application in Minera Williams
In base on the experimental evidence obtained with the sulphide precipitation study for copper and cyanide removal from the barren solution after the Merrill-Crowe process in Minera Williams. This process has been installed on a mine site at full scale. The Serpentine (flush tube) system is a viable technology for the recovery of copper, silver and subsequent recovery of HCN gas by scrubbing in NaOH. A simplified process flow diagram in which uses sodium sulphide (Na2S) such as sulphide ions, to precipitate copper and silver and convert cyanide to HCN gas, under acid conditions (pH 3 to 4) is shown in Figure 13. Barren solution is currently fed to the Serpentin at 10 to 15 liters/second at a pH 11. At this flow rate, the precipitate of calcium sulphate (scale) would not occur. In five continuous working days the treated solution exits the circuit at a pH of 4, carrying about 0 to 10 ppm of copper and 200 ppm cyanide and is pumped to two neutralizing (pH= 7) tanks. The operation of the Serpentin produce high grade copper sulphide precipitate in the range of 40 to 55% of Cu with 130 gr/ton Ag and recoveries of cyanide of 80%. On Minera Williams the results described above the plant tests were in good agreement with the results obtained in the laboratory reactor precipitate with acid Na2S. Also, in industrial operation we used only the gravity too run the operation to clean the barren solution of heavy metals (copper, zinc, lead, cadmium and iron).
This study has been very valuable in identifying that, gold and silver values are associated with argentopyrite, pyrite, sphalerite and chalcopyrite in the Bacís concentrate. The kinetics of the direct pressure size, concentration of sodium cyanide and oxygen pressure. The advantages of the Serpentin are high precipitation rate of copper and silver (99%), compact treatment facility, relatively low operation cost, the precipitate is a saleable copper/silver product, and the main advantages is that produce high grade copper sulphide precipitate in the range of 40 to 55% of Cu with 130 gr/ton Ag and recoveries of cyanide of 80%.
This study was supported by Minera William and Bacis Mining Co. in Mexico, CONACHYT and to Tecnologico Nacional de Mexico – Instituto Tecnologico de Saltillo for the use of the facilities and amenities provided to perform this work.


