Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
José Refugio Parga Torres1*, Esteban Sánchez Valdés1, Francisco Cepeda Rodríguez2, Jesús Ventura Valdés Flores1, Gregorio González Zamarripa3 and Nelson Oshogwue Etafo1
Received: December 01, 2025; Published: December 09, 2025
*Corresponding author: José Refugio Parga Torres, Departament of Metallurgy and Materials Science, TNM-ITS. Blvd V.Carranza 2400, Col. Tecnológico, Saltillo, Coahuila, C.P.25280, México
DOI: 10.26717/BJSTR.2025.64.010001
Mexico is currently the world’s leading silver producer. The preferred method for silver and gold recovery has long been the zinc cementation Merrill-Crowe process. However, after the precious metals are precipitated with zinc dust in the filter press, the resulting cake often requires dry oxidation and acid or basic leaching dissolution to control the accumulation of zinc, copper, lead, cadmium and iron impurities in the final precipitate product, that resulting in lower quality. Due to this issue, along with the lengthy six-day operation time and high reagent costs, Electrocoagulation (EC) is a promising alternative. EC requires no chemical additions for precious metal recovery. Furthermore, a separate steel reactor tank can be used to regenerate NaCN by bubbling HCN gas into liquid sodium hydroxide, achieving 90% efficiency. This total process yields a 99% Dore product quality within one day. This innovative research work used Atomic Absorption to characterize the solid products formed at iron electrodes during the Hermetic EC Process, Powder X-ray Diffraction and Scanning Electron Microscopy. The results indicate that magnetite particles and amorphous iron oxyhydroxides present in the EC products remove gold and silver and simultaneously in the chemical reactor the complex metallic ions cyanide in the pregnant rich solution was efficiently recovery and NaCN regenerate.
Keywords: Pressure Oxidation/Cyanidation; Cyanide Recovery; Air-Sparged and Electrocoagulation Reactor
The precious metals, silver and gold, have been used for thousands of years in many industrial applications, Recently, gold and silver have found increased superalloys that are formed for most new jet and rocket engines are bonded with gold brazing alloys. One new application of silver usage is in power generation and semiconductors. Today the significant increase in the value of these precious metals (Au = $ 4160 /oz, Ag = $ 52 /0z, Cu = 517 $ / lb and Pd = $ 1445 / lb) has heightened interest in the innovation recovery of these metals from their ores [1]. In Mexico extractive metallurgical operations, the used of sodium cyanide is the predominant method by which silver and gold are leaching and recovered from their ores. In metallurgical plants operation, the dissolution of gold and silver in aqueous cyanide medium is typically carried out with 0.5- 2 % NaCN, a pH of 10 – 11 and injection of air in micro bubbles allow to keep the pulp saturated with oxygen (>5ppm) and make the extraction operation more efficiency [2]. Therefore, the overall reaction for the dissolution of gold and silver in dilute, aerated, alkaline cyanide solutions may be expressed by the classic Elsner equation.

The silver leach is accomplished in the same fashion and dissolves readily in dilute cyanide solutions in the presence of oxygen. However, since silver in Mexican’s ores occurs as argentite the cyanidation and sulphide oxidation reaction [3] are as follows.
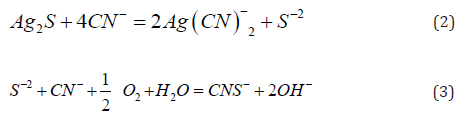
The cyanide concentration determines the rate of anodic gold dissolution while the oxygen reduction rate is dependent on the concentration of dissolved oxygen. In industrial operation, the ore materials that contain silver and gold is first delivered to a crusher. The crushed ore is them sent by truck to specially prepared leach pads. A cyanide solution of appropriate strength and pH is them evenly distributed on the heap through sprinklers an allowed to percolate through the ore. The gold and silver dissolve in the cyanide solution, which is collected in sumps and pumped to the recovery plant. Of course, high grade ores are ground and leached in agitated vessels for resident time of 72 hours, and, in some plants, gravity separation is used to recover large particles of gold liberated during milling before the pulp is cyanided [4]. Process alternatives for silver and gold recovery from alkaline cyanide solution, including solvent extraction and ion-exchange resins, have recently been reviewed and their advantages and disadvantages discussed [5-7]. The two conventional processes for gold and silver recovery from cyanide leach solution are the carbon adsorption process and the Merrill-Crowe zinc dust cementation process, in the first case, the precious metals are adsorbed onto granules of activated carbon which, after loading, are then stripped of the loaded gold and silver by a pressure and temperature and hot caustic-cyanide.
This solution is in turn fed to electrowinning cells where the gold and silver are electrolytically deposited onto cathodes of steel wool. In the second case, the Merrill-Crowe product is a filtered zinc dust precipitate. The cathodes from the carbon adsorption process or the precipitates from the Merrill-Crowe process are the melted in crucible furnaces along with fluxing materials such as borax, niter and silica. The resultant product from smelting is a Dore bullion of precious metals typically analyzing more than 90 percent precious metals. Each recovery method has advantages and disadvantages. Process selection depends on the specific conditions for a particular operation and the facilities already available. In Mexico, the Merrill-Crowe process had been the preferred process for the recovery of silver and gold. Usually in most the plants in Mexico, the zinc cake precipitate is of low quality, because lead, cadmium and especially copper ions are precipitated along with gold and silver in the filter press [8]. Therefore, chemical cementation of these base metals results in a higher consumption of zinc dust, fluxes in the smelting and refining of the precipitate and shorter life for crucibles, see in Figure 1. The effect of the copper in the cake precipitate when this is smelting with the fluxes in the induction furnace.
The comparison showed that in both plants they are metallic copper in the precipitates. The Merrill - Crowe Process in the first step clarified pregnant solution is deoxygenated by vacuum prior to zinc dust addition, where the dissolved silver and gold metal in cyanide solution is reduced to metallic state with the concurrent oxidation of zinc. The elimination of oxygen under vacuum decreases the tendency of silver and gold to redissolve in cyanide as well as decreasing the dissolution of zinc in cyanide. After this chemical reaction is completed, the solution is filtrated, and the silver and gold precipitate cake, together with impurities (Cu, Pb, Cd, Hg, Pd, Pb, etc), this dry cake is then smelted in a Dore induction furnace and poured into Dore bars. The cementation Merrill-Crowe process is preferred for very reach pregnant solutions or solutions containing large amounts of silver [8].
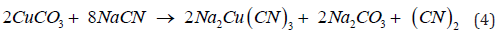
Lead minerals like galena and anglesite behave similarly toward cyanide, as the reactivity of galena largely depends on the ease with which it oxidizes to sulfate. It is important to use low concentrations of alkali; otherwise, excessive amounts of alkaline plumbite will be formed (Equation 6), which will interact with the cyanide present to form basic insoluble lead cyanide (Equation 7). With low concentrations of alkali plumbite (Pb(CN)2∙2PbO) is formed [7,10].
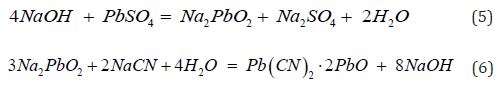
For otavite (CdCO3) where the crystals are inside of smithsonite (ZnCO3), this cadmium mineral reacts with cyanide [11] according to Equation 7.
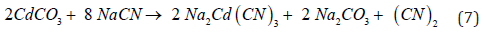
Therefore, hydrometallurgical treatment of these ores by cyanidation exhibits a series of difficulties associated with:
1. An increment in the cyanide and oxygen consumption rates,
2. A decrease in the dissolution rate of gold and silver, and
3. Principally a poor precipitate, which contains excess of copper,
lead and cadmium in the cementation process.
As a rule, the richer the pregnant solution, the better the quality of the precipitate of gold and silver, although this is modified by the presence of metals, such as copper, lead, cadmium, zinc and iron according to Parge et al, Karppinen et al, and Parga et al [12-14]. In the Merrill-Crowe process, copper is precipitated along with gold and silver. To reduce smelting costs copper can be separated from the precious metals by digesting the precipitated silver/gold/zinc in sulfuric acid and sodium hydroxide prior to smelting [14].
In cementation reactions the metal to be reduced from aqueous solution is more noble, heaving a greater electron affinity, than then precipitating [15]. For example, the recovery of metallic silver by cementation with zinc dust is an electrochemical process involving the oxidation of zinc and the reduction of the silver cyanoanion.
The overall stoichiometry for the reaction in the case of silver is as follows:
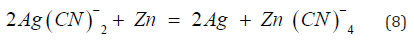
A similar reaction can be written for gold precipitation. Silver is deposited at cathodic surface site while zinc dissolves from anodic sites, and electron are conduced between the two metallic phases as shown in Figure 2, Filter Press. Most of cementation reactions are found to be first-order diffusion processes [14] with respect to the noble metal ion, and the reaction velocity constant, k, for such a reaction may be computed from the general first-order equation:

If k is not concentration dependent and the area is unchanging, Equation 11 may be integrated resulting in the integrated first- order expression.
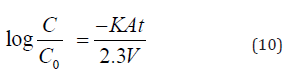
Where C and C0 are the noble metal concentrations at time t and the initial concentration at time t= 0, respectively; k is the reaction velocity constant (cm/sec); A is the reaction surface area (cm2), and V is the solution volume (cm3). In equation 9 it can be seen that the cementation rate of ions cyanide gold on zin dust is a function of the reaction area A.
In this regard the cementation reaction is analyzed in terms of the respective half cells: cathodic reduction of the precious metal (M) anion (Au(CN).
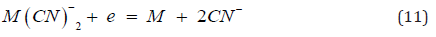
Which is coupled to the anodic dissolution of metallic zinc dust as the tetracyano-complex, by transfer of electrons through the metal.
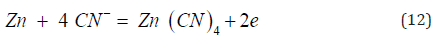
In practice, a continuous operation, the process has three filters in parallel, of which one filter is cleaning while the other two are in operation for a period of seven days.
The total cementation chemical reaction for silver is:
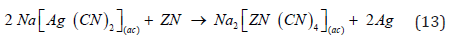
The thermodynamic properties of reactions (13) were calculated in HSC v.6 and are shown in Figure 3. It can be seen that the energy values are negative for all reactions with silver and therefore the reactions go to right side. When the cycle is completed, the precipitate inside the filter is dried with air, and then the filter press is opened to recover this precipitate that contain precious metals and impurities principally copper, lead, cadmium and mercury which are treat it with a dilute 15% sulfuric acid solution to dissolve the remaining zinc and some impurities such as copper.
Examination of samples of the filter cake from the Escalante plant in Utah, shows another interesting feature of particles from the cementation cake with respect to frame number and location in the filter press. The composition of samples for the silver cake from Frames 1, 26, and 52 are listed in Table 1, and the cake profile for each frame/sample location are displayed in Figure 4. As shown in Figure 4, the first frame was found to be about 90% full, and the filling decreased with frame number away from the front of the press with the last frame having about 20% volume filling by the silver cake. From the data in Table 1 and Figure 4, it seems that there is only a small variance in average composition from frame to frame. Specifically, the silver analysis systematically decreases from the first frame (Number 1) to the last frame (Number 52); further it is evident that the copper content in the last frame is significantly greater than that found in other two frames. In each frame the middle part of the cake contains more impurities with less silver compared to the upper bottom portions of the cake.
Increasingly stringent limitations on the purity of the Dore for the refining process have created a growing interest in the development of a novel process for the removal of lead and cadmium hydroxides from the pregnant cyanide solution before cementation in the filter press [16]. There are several methods for the removal of lead and cadmium ions from wastewater: chemical precipitation (hydroxide, sulfide and carbonate precipitation) and physical treatments (ion exchange, adsorption and foam flotation). Different kinds of adsorbents were used in the last decade to remove lead from wastewater. These include green algae, natural zeolite, fly ash, saw dust, pea nut husk, cocoa pod husk, palm kernel shell charcoal, extended perlite, and carbon nanotubes. The treatment or processes for lead and cadmium removal from wastewater must be selected to remove the existing form of lead and cadmium ions [17]. Removal of lead hydroxides and cadmium cyanide ions from cyanide liquors through adsorption on cow bone powder (Ca10(PO4)6(OH)2) is a new approach for cleaning the precious precipitate from plumbite and cadmium ions. Cow bone powder (CBP) has a high removal capacity for divalent heavy metal ions like lead and cadmium and is also an economical and natural source of phosphate [18].
The experiments for sulphide precipitation were carried out at different pH under atmospheric pressure in a glass reactor. In all the tests the barren initial solution from the Merrill-Crowe plant was from the same batch (0.1 Ag mg/L, 184 Zn mg/L, 636 Cu mg/L, 4 Fe mg/L). The following conditions were also fixed: temperature 250C; stirring speed 200 rpm; 0.5 to 2 g/l Na2S and reaction time 90 seconds. The experimental results for copper, silver, zinc and iron precipitation at different pH are presented in Table 2.
The results showed that pH had a significant effect on copper cyanide removal efficiency and the best pH was about 3 to 4.5. At this pH value copper cyanide removal efficiency could be achieved above 99 %, when influent copper concentration was 636 ppm. Some black precipitates were observed in the solution samples of the pH 2 to 6 experiments; which suggested that there was copper, silver, arsenic, zinc and iron as sulphide. The presence of this sulphide is confirmed by X-ray diffraction (not shown here), also the sample collected from the tests (see Table 1), gave rise to peaks corresponding to covellite, esfalerite and pyrite. The EDX and SEM analysis with size and morphology of the solids in the experiment are shown in
The Figure 5 of the SEM confirms the excellent crystallinity of synthetic covellite (CuS) formed during sulphide precipitation process. The EDX pattern of the precipitate at different pH values is shown in Table 3. New approach for lead, zinc and copper ions elimination in cyanidation process to improve the quality of the precipitate
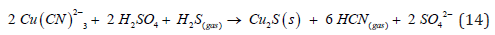
Also, if the sterile solution has a remnant of silver that was not recovered in the Merrill Crowe process, hydrogen sulfide precipitates the silver forming argentite and simultaneously the formation of 4 molecules of hydrogen cyanide that can be regenerated.
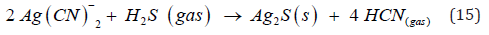
Finally, the ions of lead (Na2PbO2) and cadmium (Na2Cd (CN)3) compounds that are also in the exhausted solution can also react with hydrocyanic acid (HCN) forming precipitates of lead and cadmium sulfide and also contributing to the generation of gaseous hydrocyanic acid. According to the previous reactions for the destruction of cyanicides in an acidic medium, all the hydrogen cyanide can be recovered in a countercurrent soda adsorption tower to form sodium cyanide, patent 4097 MX [José R. Parga, Héctor A. Moreno, María T. Certuche, Jesús L. Valenzuela. Device for the regeneration of sodium cyanide in an acidic and basic medium. IMPI Utility Model 4097 MX. 2014] [19]. This regenerated product (NaCN) is then used in the initial stage of the cyanidation process to leach gold and silver. The above can be represented by the following chemical reaction carried out at a pH of 11.
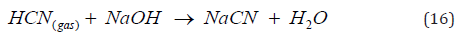
In conclusion, as in Mexico, in other countries where precipitates contaminated with copper, lead, and cadmium are produced from this electrochemical zinc cementation process, the hydrometallurgical treatment used involves leaching this calcine with sulfuric acid in an acidic medium. If the solution contains high concentrations of copper sulfate, iron cementation is employed, and the cemented copper, recovered by filtration, reaches a purity of 92% Cu. Selling this high-quality, arsenic-free copper byproduct reduces operating costs. The calcine with fewer contaminants is melted, and the resulting doré has a final purity exceeding 95%. However, the major drawback of this type of acid treatment is that it is impossible to obtain a high degree of doré purity because if lead and cadmium are present, they form insoluble sulfates in this acidic medium. When these sulfates melt with fluxes, their percentage increases due to their high affinity for gold.
The present invention is mainly concerned with the selective removal of heavy metals lead and cadmium produced by the leaching treatment of calcine from the filter press of the Merrill Crowe process, which contains them, using an acidic or basic medium as a liquid solvent. Subsequently, the leaching solution loaded with these dissolved cadmium and lead ions is treated with a filter completely filled with bovine bone as a natural adsorbent to recover these toxic metals without harming the environment (for a detailed description see patent 401381 MX: José R. Parga, José S. Parga. Hydroxyapatite filter for obtaining high-quality ions. IMPI Patent 401381 MX. 2013). This bone is primarily composed of calcium apatite, scientifically known as hydroxyapatite (HA), Ca10(PO4)6(OH)2, which is a transparent-translucent solid that is only slightly soluble in water or in a basic medium using sodium hydroxide. The ionic character of HA makes it a hard, refractory ceramic with a melting point above 1500°C. Furthermore, this ionic character allows for the partial or complete substitution of ions in the lattice by others of similar size (Pb/Cd).
Currently, worldwide in the recovery of gold and silver from cyanide solutions, the precipitate obtained from the Merrill-Crowe recovery process is contaminated with lead, cadmium, mercury, zinc, copper, and iron. This precipitate is dried and calcined, and finally, in an induction furnace with fluxes at an average temperature of 1200°C, a low-quality doré of 70% (Au/Ag) is obtained due to the aforementioned contaminants. Because of this problem, and in order to obtain high-quality doré, some hydrometallurgical plants implement additional steps to achieve this goal [20].
1. Low quality Doré is treated again with more fluxes and remove the slag more times until the desired Doré quality of 96% is achieved.
2. In the case of cadmium or lead, the treatment method is more critical and dangerous (PbOGAS), because they use an excess of NaNO3, with the aim of reducing the lead and cadmium content of the Doré in several smelting cycles.
The reaction, where adsorption occurs on the bone, which takes place in the filter packed with treated bone, is represented by the following chemical reaction:
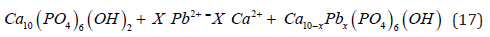
Accordingly, it can be concluded that this process of lead ion adsorption onto bone particles occurs through a diffusion-controlled process involving the toxic liquid film containing the lead ions and spreading onto the hydroxyapatite surface. Also, in general, cadmium cations are initially adsorbed onto the hydroxyapatite surface, where they replace calcium ions. This mechanism can be represented by the following chemical reaction:
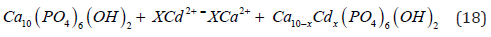
According to experimentation and analysis of the slags from the refining of DORE, the results of X-ray diffractograms and Scanning Electron Microscopy, the lead and cadmium ions in the matter are adsorbed onto the hydroxyapatite surface and then, through cationic substitution with calcium (ion exchange mechanism), the following chemical reaction for lead takes place [21]:
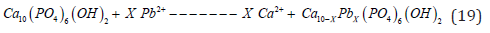
Based on this, it can be concluded that the lead adsorption process on bone particles occurs through a diffusion-controlled process involving the elements lead and cadmium on the porous surface of the hydroxyapatite [21]. Figure 4 above also shows the micrograph and EDX image of lead adsorption on the bone particles that reacted in the crucible for the purification of Doré.
The theory of Electrocoagulation (EC) is well-documented [9,22,23]. It is generally accepted that the EC process occurs in three successive stages:
1. Coagulant Formation: Electrolytic oxidation of the sacrificial electrode forms coagulant species.
2. Contaminant Destabilization: Contaminants, such as particulate suspensions and emulsions, are destabilized.
3. Floc Aggregation: The destabilized phases aggregate to form easily separable flocs.
The mechanism underlying the destabilization of contaminants, particulate suspensions, and emulsions is generally understood and can be summarized as follows [9,23-25]:
1. Double-Layer Compression: Ions generated by the dissolution of the sacrificial electrode-driven by the passage of current- interact with the charged species in the wastewater. This interaction effectively compresses the diffuse double-layer surrounding these species.
2. Charge Neutralization: Counter ions produced by the electrochemical dissolution of the sacrificial electrode neutralize the charge of ionic species in the wastewater. This neutralization significantly reduces the electrostatic interparticle repulsion, allowing van der Waals attractive forces to dominate, thus causing coagulation. This process ultimately results in a zero net charge on the particles.
3. Floc Formation and Bridging: The resulting coagulum forms flocs. These flocs create a sludge blanket that physically entraps and bridges colloidal particles that were not complexed via charge neutralization.
Note: While the general steps are established, detailed understanding of the kinetics and specific physico-chemical changes within these steps is still lacking and warrants further investigation.
The effectiveness and specific reaction pathways of EC are highly dependent on the chemistry of the aqueous medium, particularly conductivity. Other influential factors include pH, conductivity, and the concentration of chemical constituents [26]. The removal mechanisms iron (Fe), which are the most common sacrificial electrode materials used for wastewater clarification.
Electrolytic oxidation of an iron sacrificial electrode generates iron hydroxide (Fe(OH)n, where n=2 or 3). Two primary mechanisms have been proposed for the production of these coagulant species [24]:
• Mechanism 1: At the anode, iron is oxidized to Fe2+ or Fe3+ ions, which then hydrolyze in the aqueous solution to form the insoluble hydroxide precipitates.
• Mechanism 2: The OH− ions produced at the cathode migrate and react directly with the dissolving Fe at the anode to form Fe(OH)n.
• Mechanism 1
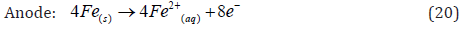
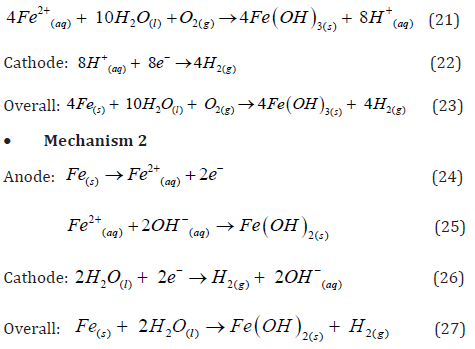
The Fe(OH)n(s) formed remains in the aqueous stream as a gelatinous suspension, which can remove the pollutants from wastewaters either by complexation or by electrostatic attraction, followed by coagulation [27]. In the surface complexation mode the pollutant acts as a ligand (L) to chemically bind hydrous iron:
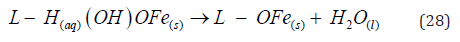
The EC experiments were conducted using a bench-scale reactor setup, a schematic of which is shown in Figure 6. The reactor consisted of a 600 ml Pyrex beaker, which served as the cell.
1. Electrodes: Two sacrificial iron electrodes were used, each measuring 10 by 2.5 cm. The separation distance between the electrodes was precisely maintained at 5 mm.
2. Power Supply: A regulated power source (Steren PRL-25 model) supplied the necessary direct current (DC) energy to the EC cell.
3. Mixing: A magnetic stirrer (Corning Model PC-310) was used to ensure continuous and uniform mixing of the solution throughout the experimental run.
When an electric current is passed through the two primary power electrodes (monopolar connection), the unpowered, intermediate conductive plates (in this case, the sacrificial electrodes) function as bipolar electrodes.
Specifically:
1. The sides of the sacrificial plates facing the anode become negatively charged (cathode sides).
2. The sides facing the cathode become positively charged (anode sides).
This arrangement means the plates have opposite charges on their parallel sides, inducing electrochemical reactions on both surfaces [3,4].
To determine the optimum operating parameters for the EC process in removing copper and cyanide, a series of experiments were conducted. The study systematically varied the following key process variables: Initial pH of the solution, residence time in the EC cell, and applied voltage and amperage (current). The experiments utilized a real barren solution supplied by the mining company William S.A. de C.V. The detailed starting concentrations and conditions are provided in Table 4.
The EC process results show that after 5 minutes the recovery of silver and gold the recovery is more than 96 %. Also, the behavior of the pH increased with the time because the decomposition of the water in the cathode section of the electrochemical cell Figure 7. These results show that with 2 stages the recovery’s for silver and gold are more than 98 % Tables 5 & 6.
1. The results of this study indicate that silver and gold can be
successfully adsorbed on iron magnetic species produced by
the iron electrodes in the Electrocoagulation process. Also,
this Electrochemical Technology may be used to recover the
sulfide precipitates of copper sulfide, zinc sulfide, lead sulfide
and cadmium sulfide.
2. The X-Ray Diffraction, Scanning Electronic Microscopy, techniques
demonstrate that the formed species are of magnetic
type, like lepidocrocite and magnetite, and amorphous iron
oxyhydroxide which adsorbed the silver and gold particles on
his surface due to the electrostatic attraction between both
metals.
3. The hermetic serpentine system offers multiple advantages
4. It is extremely economical as it can be built with materials
found in the plant
5. The cyanides removed from the solution are easily recovered
and, due to the effective sulfurization, produce excellent products
for sale (60% CuS) as it is an airtight system, (HCN / H2S)
gas is recovered in the form of NaCN.
6. It is easy to handle and its efficiency in eliminating cyanides is
greater than 99%
7. It does not use any energy and operates by gravity
8. The 98.5 % of gold and silver were removed in the experimental
EC reactor, and it was achieved in 5 minutes or less with a
current efficiency of 99 %.
9. Finally, all the products from the EC cell are used to make a
semiconductor.
The authors gratefully acknowledge the support of Tecnológico Nacional de México- ITSaltillo for their invaluable collaboration in this project. We also extend our deepest appreciation to the Mexican National Council of Science and Technology (CONACHYT) for their financial support and commitment to advancing scientific research in Mexico. We sincerely thank Minera Williams, Escalante Plant in USA, Minas de Basis, and Minera La Paz for their crucial backing and active participation, which were essential to the success of this work. Likewise, we sincerely thank the University of UTHA (Professor Jan D. Miller). This project would not have been possible without the joint support of these institutions and companies, whose dedication to innovation and technological development has been instrumental.


