Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Hanane Dergaoui*, Mohamed Agoujil, Yassine Marjane and Mohamed Chakour
Received: November 09, 2025; Published: November 18, 2025
*Corresponding author: Hanane Dergaoui, Hematology Laboratory, Avicenne Military Hospital, Morocco
DOI: 10.26717/BJSTR.2025.63.009963
Reticulocyte hemoglobin content (Ret-He) is an automated hematological parameter reflecting iron availability for erythropoiesis. It is a promising alternative to conventional iron studies for diagnosing iron deficiency anemia (IDA) and monitoring therapeutic response. The aim of our study was to evaluate the usefulness of Ret- He in monitoring adults with iron deficiency anemia treated with oral iron. Thirty patients were included and followed using complete blood counts performed on Sysmex XN-1500 and Siemens Atellica Solution analyzers. All patients received two tablets of ferrous sulfate (80 mg) every other day for three months, with assessments on day 8, month 1, and month 3. The mean age was 40.56 ± 17.45 years, with a clear female predominance (sex ratio = 0.25). Fatigue was the most common symptom. Digestive causes predominated, particularly Helicobacter pylori infection (50%). Initial parameters showed a mean hemoglobin level of 8.61 g/dL, reticulocyte count < 120,000/μL, mean Ret-He of 20.89 pg, and low ferritin (3.87 ng/mL). Follow-up revealed that an increase in Ret- He as early as the first week (reticulocyte crisis) was a significant predictor of treatment response (p = 0.0001). In comparison, ferritin elevation appeared only after one month. Ret-He increased steadily throughout the follow- up period, correlating with improvements in hemoglobin levels. In conclusion, Ret-He emerges as an early, reliable, and easily accessible biomarker for both diagnosing iron deficiency anemia and assessing the efficacy of oral iron therapy.
Keywords: Iron Deficiency Anemia; Reticulocyte Hemoglobin Equivalent (Ret-He); Early Diagnosis; Therapeutic Monitoring; Oral Iron
Iron deficiency is the most common nutritional deficiency worldwide and remains the leading cause of anemia. It can progress silently: initially, hemoglobin remains normal despite decreased iron stores, explaining why iron deficiency can exist without anemia. During this phase, only serum ferritin and transferrin saturation are reduced. When stores are completely depleted, hemoglobin levels drop, defining iron deficiency anemia. The gold standard for diagnosis is Perl’s staining (Prussian blue staining) on a myelogram, which evaluates iron deposits in macrophages and erythroid precursors. However, this method is invasive and costly, limiting its use in routine practice. Biochemical markers are more commonly used: serum ferritin, serum iron, total iron-binding capacity (TIBC), and saturation coefficient (SC). Ferritin is a good indicator of iron stores: low values indicate deficiency. However, as an acute-phase protein, it can be normal or elevated in cases of inflammation, infection, or malignancy, complicating interpretation. Serum iron decreases in iron deficiency but shows significant variations depending on dietary intake and circadian rhythm. TIBC and SC, calculated from transferrin, are also influenced by variations in transferrin levels. For earlier detection, measuring reticulocyte hemoglobin content (Ret-He) is useful. Reticulocytes, the immediate precursors of red blood cells, more rapidly reflect iron metabolism status compared to classic indices such as Hb, MCV, MCHC, or the percentage of hypochromic red blood cells (%Hypo-He), which change only later due to the prolonged lifespan of erythrocytes (~120 days).
The aim of this study was to evaluate the usefulness of the Ret-He parameter in the diagnosis and monitoring of patients with iron deficiency anemia treated orally, comparing it with classical hematological and biochemical markers. This was a prospective interventional study conducted at Avicenne Military Hospital in Marrakech over an eight-month period (01/04/2024 – 01/11/2024). A total of 42 cases of iron deficiency anemia were included, representing 45.6% of iron deficiency anemias recorded during this period. Twelve patients were lost to follow-up, leaving a final sample of 30 patients (37.03% of cases) for analysis. Consultations were conducted in the clinical hematology department, while laboratory tests were performed in the hospital’s laboratory. The analytical process consisted of three steps: a pre-analytical phase including clinical data collection, blood sampling in EDTA and dry tubes, and transport and storage of samples; an analytical phase corresponding to technical execution on the analyzers, preceded by quality control checks; and a post-analytical phase ensuring validation and communication of results. The hematological workup included a complete blood count, erythrocyte indices (hemoglobin, MCV, MCHC, MCH), reticulocyte count, and reticulocyte hemoglobin content (Ret-He). Analyses were performed on the Sysmex XN-1500 analyzer, allowing for a complete blood count analysis and measurement of Ret-He by flow fluoro-cytometry. The reference range was 28 to 35 pg, with values below 28 pg suggesting iron deficiency.
The biochemical workup, performed on the Atellica Solution analyzer (Siemens), included measurements of ferritin, serum iron, and transferrin, allowing for the calculation of the saturation coefficient and total iron-binding capacity. The study involved several successive consultations. The first consultation included a questionnaire (identity, medical history, diet, functional signs), a clinical examination, and an initial workup (CBC, reticulocytes, Ret-He, ferritin, CRP, SC, TIBC, and vitamin workup depending on the context). The second consultation, scheduled one week later, involved interpreting the results, verifying inclusion criteria, and obtaining informed consent. Treatment with ferrous sulfate (80 mg, two tablets every other morning for three months) was prescribed, along with a control workup at ten days (CBC, reticulocytes, Ret-He). The third consultation, corresponding to the first control after eight days of treatment, assessed clinical tolerance and interpreted the results of the workup, then indicated a onemonth control (CBC, ferritin, CRP). The fourth consultation, after one month of treatment, included clinical and biological reassessment, as well as the prescription of the final workup at three months (CBC, ferritin, serum iron, TIBC, SC, CRP). Finally, the fifth consultation, after three months of treatment, allowed for the final evaluation of the results. Data entry and analysis were performed using Word and Excel 2018 software. Qualitative variables were expressed as percentages, quantitative data as mean ± standard deviation, and Student’s t-test was used to compare variables, with significance set at p < 0.05. Ethically, the study objectives were clearly explained to all participants, who gave their informed consent. Confidentiality and protection of personal data were strictly respected.
The mean age of the patients was 40.56 ± 17.45 years (Figure 1). Women accounted for the majority of cases (83%, 24 cases), compared to 6 men (17%), with a male-to-female sex ratio of 0.25 (Figure 2). Among the women, 67% were of childbearing age and 16% were menopausal. Maritally, 57% of patients were married and 43% were single (Figure 3). The majority came from urban areas (77%), compared to 23% from rural areas. Clinically, anemia was most often discovered during a routine check-up (40%), followed by referrals from a specialist (30%), a general practitioner (16.6%), or via the emergency department (13.4%) (Table 1). The medical history was dominated by menorrhagia (23.3%), followed by recent surgery (20%), digestive bleeding, and NSAID use (10%). Metrorrhagia, repeated blood donations, closely spaced pregnancies, and a history of anemia each accounted for 6.6%, and geophagia for 3.3% (Figure 4). The main functional signs reported were dominated by fatigue, exertional dyspnea, phanere disorders, followed by headaches, palpitations, tinnitus, exertional angina, and dizziness (Figure 5). Physical signs were dominated by cutaneous-mucosal pallor, tachycardia, and brittle nails (Figure 6). Biologically, the mean hemoglobin level was 8.61 g/dL, MCV 69.57 fL, MCH 20.89 pg, and MCHC 29.06 g/dL (Figure 7). The reticulocyte count ranged from 24,400/μL to 112,000/ μL, always <120,000/μL. The mean Ret-He was 20.89 pg, below 28 pg in all patients. The iron workup showed a mean ferritin of 3.87 ng/ mL, serum iron of 4.58 μmol/L, transferrin of 3.47 g/L, TIBC of 86.06 μmol/L, and SC of 5.74% (Figure 8).
Regarding etiology, gynecological exploration found a cause in 10 out of 15 cases (functional menorrhagia, closely spaced pregnancies, IUD, fibroid uterus). Digestive exploration showed H. pylori gastritis in 50%, NSAID gastritis (10%), hemorrhoids (10%), erosive enteritis (3.3%), and geophagia (3.3%); explorations were normal in 23.3% (Table 2). Overall, digestive causes accounted for 76.6%, compared to 33.3% for gynecological causes. All patients received oral treatment with ferrous iron 160 mg/day, every other day, for 3 months, combined with etiological treatment. Biological evolution showed a progressive increase in hemoglobin and Ret-He: after 8 days, mean Hb was 9.27 g/dL and Ret-He was 24.9 pg (Table 3); after 1 month, Hb was 10.95 g/dL and Ret-He was 27.9 pg (Table 4); after 3 months, Hb was 12.6 g/dL and Ret-He was 31.7 pg, with a mean ferritin of 27.7 ng/mL (Table 5). According to the criteria of (Moretti, et al. [1,2]), 93% of patients were responders (≥ 2 g/dL gain in 4 weeks), while 2 patients (7%) were non-responders. Ret-He proved to be an early and predictive marker of treatment response as early as the first week (p=0.001), unlike Hb (p=0.6) (Figure 8). A linear correlation was observed between the increase in Ret-He and that of Hb: each additional unit of Ret-He corresponded to a gain of 0.36 g/dL of Hb (Table 6). Finally, the comparison between Ret-He and the iron workup showed that ferritin only increased significantly after one month, unlike Ret- He, which increased early and linearly (Figure 8).
Iron deficiency anemia is defined by a decrease in iron stores leading to a drop in hemoglobin concentration; erythrocytes are often hypochromic and microcytic. Patho physiologically, iron is distributed between a metabolically active pool and storage pools; total body iron is about 3.5 g in men and 2.5 g in women, with a difference due to size and menstrual losses. The distribution of body iron is as follows: hemoglobin 2 g (men) / 1.5 g (women), ferritin 1 g / 0.6 g, hemosiderin 300 mg, myoglobin 200 mg, tissue enzymes 150 mg, and transport compartment 3 mg. Deficiency progresses in stages: first depletion of medullary reserves (deficiency stage), then impairment of erythrocyte synthesis, and finally iron deficiency anemia; severe and prolonged deficiency can lead to cellular enzymatic dysfunction. Clinically, the body implements compensatory mechanisms: increased tissue oxygen extraction (from ~25 → 60%) and increased heart rate and stroke volume to maintain perfusion and oxygenation. The clinical picture depends on the severity and speed of onset. Signs related to tissue hypoxia include pallor, fatigue, exertional dyspnea, myocardial ischemia, headaches, dizziness, and syncope; the hyperdynamic state can manifest as palpitations, bounding pulse, cardiac murmur, and tinnitus; hypovolemia can cause hypotension, oliguria, or shock. Etiologically, the major cause is chronic blood loss: occult digestive bleeding (ulcer, cancer, hemorrhoids, ectasia), parasitic infections (hookworm) in low-resource countries, and in non-menopausal women, significant cumulative menstrual losses.
Other losses (urinary, pulmonary) or intravascular hemolysis can be involved. Increased needs (growth, adolescence, pregnancy, lactation) favor deficiency. Decreased absorption (gastrectomy, celiac disease, atrophic gastritis, H. pylori infection, achlorhydria, short bowel) or rare entities such as IRIDA (TMPRSS6 mutations) explain other cases. Management always requires etiological investigation before treatment. Oral supplements (sulfate, gluconate, fumarate, or iron saccharate) are preferred as first-line treatment, taken preferably 30 minutes before meals. Usual dose: 65 mg of elemental iron (e.g., 325 mg ferrous sulfate) 1×/day or on alternate days; higher doses are poorly absorbed (increased hepcidin) and increase digestive side effects. Oral treatment should be continued for ≈6 months until reserves are replenished (ferritin). Parenteral iron (iron sucrose, ferric carboxymaltose) provides a faster response but carries risks of infusion reactions; carboxymaltose allows administration of 1000 mg in a single infusion (practical when rapid repletion is needed). Indications include oral intolerance, malabsorption, urgent needs, continuous blood loss, hemodialysis in renal failure, etc. The total dose is generally calculated based on weight and Hb (1000 mg often sufficient), and the iron workup should be reviewed after ~4 weeks. Typically, Hb increases by 0.7–1 g/week; anemia should be corrected in ≈2 months; an insufficient response suggests persistent bleeding, infection, cancer, or insufficient intake/malabsorption.
For exploration, Perl’s staining on bone marrow smear remains the gold standard for evaluating iron reserves (sideroblasts), but it is increasingly less used. The hemogram: complete blood count and smear, as well as reticulocytes, are useful for guiding toward iron deficiency anemia and other differential diagnoses. Among the biochemical parameters, serum iron varies with diet and circadian rhythm and is not reliable alone; serum ferritin reflects reserves but increases in inflammation. Transferrin, as well as total iron-binding capacity (TIBC) and saturation coefficient (SC ≈30% physiologically) complete the workup. Soluble transferrin receptor (sTfR) is a marker of iron bioavailability independent of inflammation. Reticulocyte hemoglobin equivalent (Ret-He)—available on new-generation analyzers (e.g., Sysmex XN)—decreases early in deficiency and allows for early diagnosis and monitoring, before the elevation of classic Hb. In published series, the mean age of patients varies between ~33 and 44 years depending on the studies; our series: 40.56 ± 17.45 years, consistent with regional series. Women of childbearing age are the most affected; the male-to-female sex ratio is most often <0.6; in our series, it was 0.25, consistent with the female predominance linked to menstruation, pregnancies, and socioeconomic factors. Clinically, the most frequent histories are menorrhagia (23.3% in our series), also found in other studies. The dominant signs in the series are fatigue and cutaneous- mucosal pallor; many anemias remain discovered fortuitously.
In our cohort, fatigue 100%, exertional dyspnea 93.3%, pallor 90%. Hematological results show a mean Hb generally between 7 and 9.6 g/dL depending on the series; in our series, the mean Hb value was 8.61 ±1.58 g/dL, MCV 69.57 fL, MCH 20.89 pg. Ret-He varies between ≈20 and 27 pg; in our series, the mean Ret-He value was 20.89 pg, comparable to that reported by (M Uçar, et al. [3-4]). The diagnostic performances of Ret-He reported in the literature are generally high. Biochemically, the iron workup showed a mean ferritin of 3.87 ng/mL, serum iron of 4.58 μmol/L, transferrin of 3.47 g/L, TIBC of 86.06 μmol/L, and SC of 5.74%. These results are consistent with national and international literature data. Etiologically, digestive and gynecological causes remain predominant. Studies report 48–64% digestive causes and 25–55% gynecological causes, while a proportion of cases remain without specified etiology. In our series, digestive causes were predominant (76.6%), dominated by H. pylori gastritis (50%), followed by gynecological causes (33.1%), mainly functional menorrhagia (13.3%), confirming the link between H. pylori infection and iron deficiency documented by other studies. Therapeutically, management modalities vary according to studies. Some authors recommend the administration of ferric carboxymaltose intravenously (1 g in 1 to 2 infusions), others recommend IV iron-sucrose, while oral treatment remains a preferred alternative in moderate forms. Our approach (oral treatment with 3-month follow-up) is similar to some Indonesian and Turkish series.
Regarding evolution, Ret-He proves to be an early and predictive marker of therapeutic response. (Uçar, et al. [3-4]). reported high specificity (90–96%) and comparable sensitivity for this parameter. According to Uçar, the absence of a difference in Hb value on the 5th day is compensated by an early elevation of Ret-He, reflecting a rapid medullary response to iron supplementation. Auebach also showed that the initial Ret-He value predicted the Hb response after IV treatment. In our series, the 3-month follow-up confirmed the prognostic value of Ret-He. As early as the first week, the increase in Ret-He was significant (p=0.0001), preceding that of Hb. Ferritin did not increase significantly until after the first month, confirming that Ret-He, which evolves in parallel with reticulocyte production, is an earlier indicator of therapeutic response (Figures 9-12) (Tables 7-10). Our study is among the first to have followed the evolution of Ret-He over 3 months, in comparison with standard iron workup parameters [5- 29].
Table 10: Diagnostic Performance of Ret-He in Different Studies in Patients with Iron Deficiency Anemia.
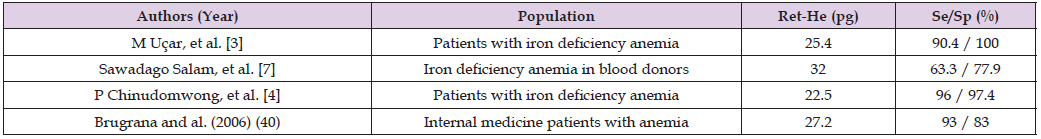
Iron deficiency anemia remains a major global public health problem, particularly in developing countries, where it mainly affects young children and women of childbearing age. Our results suggest that reticulocyte hemoglobin equivalent (Ret-He) is an early, reliable, and sensitive marker of response to iron therapy, particularly oral therapy, in patients with iron deficiency anemia. Beyond its biological relevance, Ret-He has the advantage of being a simple, rapid, and economical tool, usable in routine practice to confirm or exclude iron deficiency and to monitor therapeutic efficacy. However, confirmation of these results requires prospective randomized studies, including larger cohorts, to validate the place of Ret-He in the diagnostic strategy and therapeutic monitoring of iron deficiency anemia.


