Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jiangnan Feng1*, Zhenghua Ma1, Chengsheng Yi1, Xia Liu1, Meiying Gao2, Dengbin Zhu1, Minghai Ma1, Rui Cheng3, Xue Feng1 and Yu Yan1
Received: October 29, 2025; Published: November 13, 2025
*Corresponding author: Jiangnan Feng, Tongrun Tang TCM Clinic, Nanchong City, Sichuan, China
DOI: 10.26717/BJSTR.2025.63.009957
The real-world treatment outcomes of the herbal remedy Marecipe AV for feline chronic gingivostomatitis (FCGS) associated with feline calicivirus (FCV) infection are presented. The treatment involved the oral administration of Marecipe AV as monotherapy. These outcomes included clinical remission as well as changes in viral load both before and after treatment. All 62 cats treated with Marecipe AV monotherapy achieved clinical remission, resulting in a treatment response rate and clinical remission rate of 100%. In all treated cases, the clinical signs and symptoms induced by FCV, such as drooling and tearing, achieved complete remission within 7 to 15 days of treatment. A significant reduction in viral load was observed on day 7 after treatment (mean Ct 30.49 compared to a baseline of 23.90) in all treated cases. By day 15, the mean Ct was 33.87 (close to the detection limit). High recurrence rates were noted; specifically, in mild to moderate cases, the recurrence rate reached 83.33% at three months. In the group that underwent three retreatments with Marecipe AV, the recurrence rates at 3 months and 6 months were both 28.57%. The Marecipe AV herbal remedy is an effective treatment for FCGS in cats, resulting in a remarkable 100% clinical remission. However, the associated recurrence rates are high, necessitating repeated treatments for many cured cats.
Keywords: Feline Chronic Gingivostomatitis (FCGS); Feline Calicivirus (FCV); Herbal Medicine; Virus; Viral Infectious Diseases
Abbreviations: FCGS: Feline Chronic Gingivostomatitis; FCV: Feline Calicivirus; TCM: Traditional Chinese Medicine; qPCR: Quantitative Polymerase Chain Reaction; CT: Cycle Threshold; ASIS: Alveolar Stomatitis Intensity Score: EOT: End of Treatment: COPS-C/F: Composite Oral and Maxillofacial Pain Scale Canine/Feline
Feline chronic gingivostomatitis (FCGS) is a severe inflammatory syndrome. The predominant cause of FCGS is oral infection with feline calicivirus (FCV) rather than bacterial infection. FCGS is a frustrating condition for clinicians because various reported treatments for FCGS are unrewarding, none of these treatments has become a definitive solution. Euthanasia is sometimes considered for cats that do not respond to treatment [1-10]. Feng and colleagues recently reported a groundbreaking therapy for the treatment of acute fatal viral infections. An herbal remedy known as Maresipe AV has shown significant therapeutic effects against acute lethal viral infections in both animals and humans. In this study, we present the outcomes of the Maresipe AV herbal remedy in treating chronic viral infectious diseases, including cases of FCGS in cats, within a real-world setting [11-14].
The treatment modality for Marecipe AV herbal therapy involves oral administration. Ma’s GWO is a therapeutic ointment used for nonhealing wounds in our clinic. In this study, it was used as an adjuvant therapy alongside Marecipe AV to promote the healing of oral ulcers. Viral detection and quantification of the viral load were performed via real-time quantitative PCR (qPCR) [10,15,16]. Efficacy was evaluated by comparing the alveolar stomatitis intensity score (ASIS) before the treatment period and at the end of the treatment (EOT) period. Clinical remission is characterized by the complete healing of oral lesions, with the exception of hyperplastic lesions. More specifically, a final score of either 0 or 1 on the ASIS scale at the end of treatment (EOT) is indicative of clinical remission. In most cats affected by ACGS, the complete regression of proliferative lesions could require a substantial period or might not occur at all. Given this finding, proliferative lesions were excluded from the assessment of the short-term efficacy of clinical remission [17]. The secondary outcome was the FCV load, which was used to evaluate changes in the viral load in the cats before and after treatment. Statistical analyses were conducted via the Statistical Package for the Social Sciences (SPSS) version 27 for Windows.
In this study, treatment was conducted in accordance with the daily operational practices of traditional Chinese medicine (TCM). Licensed TCM doctors prepared prescriptions, which the nursing staff used to procure the necessary herbs from the pharmacy. The Marecipe AV herbal medicine has been in use for over a hundred years in this way. This approach adhered to the laws, regulations, and ethical guidelines governing TCM. In addition, this study used feral cats with ACGS rather than experimental animals. Although the research methods for treating feral cats in the real world adhered to the standard working procedures of TCM and complied with traditional norms and ethical standards, we submitted an application for ethical approval, which was granted. The Ethics Committee of TCM at Tongren Tang Clinic approved this study.
A total of 36 cats, comprising 24 females and 12 males aged between 1 and 9 years, were included in the study. All treated subjects were feral cats captured from three districts. Among these 36 cats, 21 received Marecipe AV monotherapy more than twice. Finally, 40 patients were classified as having mild-to-moderate ACGS, whereas 22 cats were classified as having severe ACGS. Upon admission, 58 cats tested positive for FCV, 3 cats tested positive for both FCV and herpesvirus, and 1 cat tested positive for herpes simplex virus. Forty cats with mild to moderate oral inflammation associated with ACGS received monotherapy with orally administered Marecipe AV for 7-15 days. In all treated cases, either severe or mild to moderate ACGS, the drooling symptoms resolved within 2 to 5 days after the initiation of treatment. A full return to normal eating and activities occurred within 7 to 15 days. Oral examinations revealed no redness or near-normal coloration of the oral mucosa, along with complete healing of the ulcers. The hyperplastic lesions exhibited slight shrinkage following the complete resolution of redness and swelling. The ASIS scores were EOT 0 or EOT 0 1 in all the cases, and the Ct value of the FCV was greater than 33. These cases were deemed clinically cured by veterinarians and were subsequently discharged.
Twenty-two cats presenting with severe ACGS were treated with monotherapy using Marecipe AV. To achieve clinical remission, the average treatment duration was 63.5 days (range: 45–85 days). A prolonged treatment period was required because the ulcers did not heal, although other ACGS symptoms had resolved after 15 days of treatment. The combined treatment of Marecipe AV and Ma’s GWO significantly reduced the treatment duration, decreasing it from 63.5 days with monotherapy to 19.6 days (range: 15-22 days) with combination therapy (Table 1).
Table 1: Clinical outcomes of Marecipe AV therapy in the treatment of chronic gingivitis-stomatitis in calicivirus-positive cats.
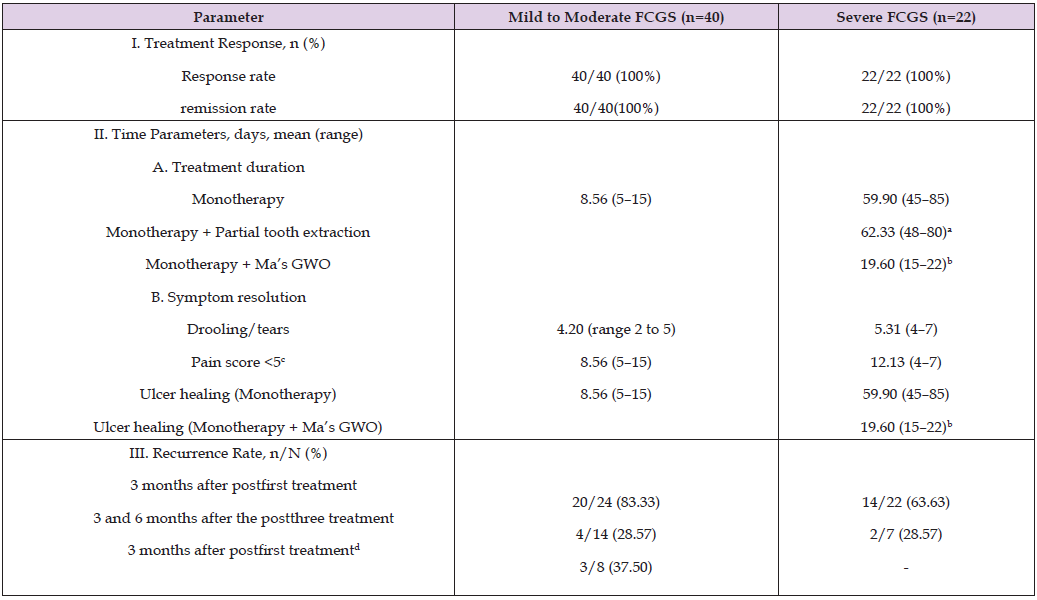
Note: Footnotes: Data are presented as the n (%) or mean (range). a Subgroups n=6 for each intervention; b Combination therapy; c Normalized feeding behavior (Feline Pain Scale 0--10); d Total number of cats housed in FCV-free environments after the treatment. This table summarizes the outcomes of treating 62 cats with varying severities of Feline Chronic Gingivostomatitis (FCGS) via the Marecipe AV. The results are categorized into two groups: mild-to-moderate cases (n = 40) and severe cases (n = 22). Response and remission rate: All 62 cats achieved a 100% response, and the clinical remission rate duration and symptom resolution were as follows: 1. Monotherapy: For mild-to-moderate FCGS, clinical remission occurred in an average of 8.56 days (range: 5--15 days). For severe FCGS, treatment lasts an average of 59.9 days (range: 45--85 days), primarily due to challenges in healing oral ulcers.2. Combination Therapy (Marecipe AV + Ma’s GWO Ointment): In severe cases, this combination reduced the healing time to an average of 19.6 days (range: 15--22 days). Combination of Monotherapy and Partial Extraction: The average treatment duration was 62.33 days, similar to Marecipe AV alone. Recurrence rate: The 3-month recurrence rate after a single treatment was 83.33% in cats with mild-to-moderate FCGS and 63.63% in those with severe FCGS. The posttreatment recurrence rate was 28.57% among all the treated cats.
All cats undergoing treatment were subjected to viral quantification via quantitative polymerase chain reaction (qPCR). To monitor changes in viral load, the ten cats serving as negative controls did not begin treatment immediately after feline calicivirus (FCV) testing. Instead, they commenced Marecipe AV treatment 20 days post-diagnosis. The dynamics of viral load in FCV-positive cats with FCGS following Marecipe AV treatment were as follows: In the treated group (n = 62), the mean cycle threshold (Ct) values were 23.90 on Day 0, 30.49 on Day 7, 33.87 on Day 15, and 34.63 on Day 75. Complete viral clearance (Ct = 0) was achieved in three cats after an extended treatment period exceeding 45 days.In the untreated control group (n = 10), the mean Ct values were 22.63 on Day 0 and 23.52 from Day 20 to Day 25. According to the manufacturer’s guidelines, Ct values ≤ 25 indicate a high viral load; values > 33 to ≤ 35 suggest a very low viral load (approaching the lower limit of detection). The treated cats were released to their original living areas after being cured and discharged from the hospital. Among 36 of the 40 treated cats, relapses occurred after discharge, with a mean recurrence time of 38.8 days (ranging from 20-85 days). The recurrence rate at three months post-treatment was 83.33%. In contrast, the three-month recurrence rate was 63.63% among 14 cats with severe FCGSs who were treated for more than 45 days. Three months post-treatment, the recurrence rate among 8 cats housed in FCV-free environments was 37.5%. High viral loads were detected in most recurrent cats, including cats that lived in FCV-free environments. Fourteen cats received three sessions of Marecipe AV monotherapy. The recurrence rate during the 3- and 6-month observation periods following complete remission was 28.57%.
As with other chronic viral infectious diseases, there is still no satisfactory treatment for ACGS. The current peer-reviewed literature on FCGS therapeutic outcomes has demonstrated the statistical success rates of various treatments [18]. Given the demonstrated potent efficacy and virus-clearing capabilities of the Marecipe AV herbal remedy in various viral infectious diseases, we hypothesized that reducing or eradicating feline calicivirus (FCV) in the oral mucosa could attenuate the associated immune response, thereby achieving the therapeutic objective for feline chronic gingivostomatitis syndrome (FCGS). Consequently, Marecipe AV was utilized to treat FCGS in a real-world setting, in accordance with TCM ethics and regulations. Marecipe AV herbal remedies have demonstrated significant therapeutic efficacy in treating feline chronic gingivitis-stomatitis (FCGS) in cats positive for feline calicivirus. All 62 cats achieved clinical remission, with no instances of treatment failure, and the complete remission rate reached 100%. This outcome is notably higher than the currently reported complete remission rates of 28.4-41% for full-mouth dental extraction, which is the current gold standard of care for FCGS [15]. These results indicate that the Marecipe AV herbal remedy has strong pharmacological effects on managing inflammation related to FCV.
In both mild ACGS and severe ACGS, oral inflammation was well controlled after 2 to 5 days of oral administration. Following just 7 to 15 days of oral administration, mild to moderate ACGS can be completely cured. Although monotherapy with the Marecipe AV remedy for severe ACGS requires long-term treatment cycles of 45-85 days to achieve clinical remission, the observed prolonged treatment duration is due primarily to the challenges associated with healing oral ulcers. To expedite the healing process, Ma’s GWO was applied to the ulcer surfaces during monotherapy with Marecipe AV Remedy. This combination therapy reduced the treatment period from 45--85 days to approximately 20 days. Marecipe AV herbal medicine has demonstrated effectiveness in inhibiting viral activity in vivo. The significant reduction in viral load from high to very low levels, approaching the lower limit of detection, after only 7 to 15 days of oral administration indicates a direct viral clearance effect of Marecipe AV. However, in the majority of treated cases, feline calicivirus (FCV) can still be detected without complete viral clearance, although the viral content is extremely low. These findings suggest that Marecipe AV herbal medicine may induce viral suppression rather than complete elimination of the virus. In this study, 37% of the cats that were cured and subsequently placed in a virus-free environment experienced a relapse shortly after discharge, with a high viral load detected in these recurrent cases.
This suggests that the reemergence of a high viral load may be due to residual viral particles that were not effectively cleared from the host organism, rather than reinfection in a viral-contaminated multi-cat environment. Recurrence, particularly during short-term treatment, is a significant limitation of Marecipe AV therapy in cats with ACGS. The recurrence rate was 83.33% in treated cats with mild to moderate ACGS. For cats experiencing recurrent ACGS, multiple interventions using Marecipe AV herbal medicine have been shown to be beneficial. The recurrence rate after three treatments was 28.57%, which was markedly lower than the initial rate. Nevertheless, A considerable number of cats with ACGS will likely require lifelong treatment with the Marecipe AV herbal remedy.
This study has several limitations. First, the lack of data on treatment cycles beyond 3 months prevents the assessment of whether longer treatment durations lead to the complete clearance of feline calicivirus (FCV) from the body. Second, the sample size of cases involving multiple treatments (specifically, more than three applications) of Marecipe AV is insufficient for a comprehensive evaluation of the long-term efficacy of multiple treatments on recurrence. Third, no data are available for the combined treatment of full-mouth extraction and Marecipe AV. Considering that full-mouth extraction can effectively remove residual tooth roots in gums, which act as reservoirs for feline caliciviruses, a combined treatment strategy may be more conducive to thoroughly eradicating the virus and prolonging the recurrence interval.
Marecipe AV herbal remedies can effectively treat feline calicivirus (FCV)-associated feline chronic gingivostomatitis (FCGS) in cats, achieving a 100% clinical remission rate. However, high short-term recurrence rates have been observed in cats treated with Marecipe AV. A significant number of cats that have been cured require multiple treatments to effectively manage their condition.
Not applicable.
Zhenghua Ma, Jiangnan Feng and Chengsheng Yi conceptualized the study, designed the methodology, and supervised the research. Zhenghua Ma, Jiangnan Feng, Chengsheng Yi and Xia Liu performed the experiments and data collection. Meiying Gao, Dengbin Zhu, and Minghai Ma contributed to the data analysis and interpretation. Rui Cheng and Xue Feng provided critical resources and technical support. Yu Yan assisted in manuscript preparation and revision. All the authors reviewed and approved the final manuscript.
This study received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
The datasets generated and analyzed during the study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate. The study protocol was approved by the research ethics committee at each participating center: the Research Ethics Committee of TCM at Tongren Tang Clinic approved this study (Ethics Committee document Nos. 202101 and 202501). This study used feral cats with ACGS rather than experimental animals. Although the research methods for treating feral cats in the real world adhered to the standard working procedures of TCM and complied with traditional norms and ethical standards, we submitted an application for ethical approval, which was granted. The Ethics Committee of TCM at Tongren Tang Clinic approved this study, and the requirement for written informed consent was waived. This study was also approved by the ethics committees.
Not applicable.
The authors declare that they have no competing interests.
Not applicable.


