Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Kyosuke Goto1,2, Yutaka Shigemori1,3, Kentaro Masuda1, Nana Otsuka3 and Muneyuki Tachihara1
Received: October 16, 2025; Published: October 27, 2025
*Corresponding author: Yutaka Shigemori, Graduate School of Sports and Health Science, Fukuoka University, Department of Rehabilitation, Fukuoka University Hospital, Fukuoka 814-0180, Japan
DOI: 10.26717/BJSTR.2025.63.009930
Recurrent Lateral Ankle Sprain (RLAS) is a common sequela of ankle injury that may lead to chronic ankle instability (CAI). This study investigated superficial sensory function in athletes with RLAS using Quantitative Sensory Testing (QST). Eighteen first-division female basketball players (11 RLAS, 6 controls) 1 excluded due to meeting the exclusion criteria underwent Current Perception Threshold (CPT) and Pressure Pain Threshold (PPT) assessments at the Anterior Talofibular Ligament (ATFL) and Tibialis Anterior (TA) regions. CPT and PPT at the ATFL region were significantly higher in the RLAS group than in controls (p < 0.01), while no differences were found at the TA region. Among RLAS ankles, those with ≥5 sprains showed higher ATFL CPT values than those with 2–4 sprains (p < 0.05), suggesting cumulative sensory decline. These findings indicate localized hypoesthesia and elevated mechanical thresholds around the ATFL due to repetitive ligamentous and neural injury. RLAS may involve both peripheral desensitization and central neuroplastic adaptation. Incorporating objective sensory assessments such as QST may improve diagnosis and guide individualized rehabilitation to prevent progression toward CAI.
Keywords: Recurrent Lateral Ankle Sprain; Chronic Ankle Instability; Quantitative Sensory Testing
Abbreviations: RLAS: Recurrent Lateral Ankle Sprain; LAS : Lateral Ankle Sprain; CAI: Chronic Ankle Instability; QST: Quantitative Sensory Testing; CPT: Current Perception Threshold; PPT: Pressure Pain Threshold; TA: Tibialis Anterior; ATFL: Anterior Talofibular Ligament; AS: Ankle Sprain; ICC: Intraclass Correlation Coefficients; CAIT: Cumberland Ankle Instability Tool; SPN: Superficial Peroneal Nerve; PPT : Pressure Pain Thresholds
Ankle Sprain (AS) is among the most common musculoskeletal injuries, accounting for approximately 20% of all sports-related injuries [1]. Lateral Ankle Sprain (LAS) is the most frequent subtype, typically resulting from a forced inversion mechanism during landing or cutting and injuring the lateral ligament complex. Although conservative treatment is considered first-line, the recurrence rate of LAS remains high [2]. Up to 32% of patients fail to achieve full recovery and progress to Chronic Ankle Instability (CAI), and as many as 70% experience persistent functional deficits [3]. Moreover, up to 50% of adults with a history of recurrent LAS report ankle problems persisting for more than 10 years [4]. Despite extensive clinical observation, the neurophysiological mechanisms underlying recurrent LAS and CAI are not fully understood. Among the lateral ligaments, the Anterior Talofibular Ligament (ATFL) is most commonly injured in LAS; in more severe cases, the calcaneofibular ligament may also be involved [5]. Post-injury, pain, swelling, and inflammation are prominent, but subtle changes in sensorimotor function and psychological or affective responses may also occur. The interaction of these factors can foster functional deficits that contribute to the development of CAI and delay optimal tissue healing. In addition, inflammatory processes and the release of pain mediators in injured tissues may impair somatosensory function and alter motor output strategies.
Through inflammatory, neural, and endocrine pathways, these reactions can induce local edema and neuromuscular inhibition at Central Nervous System Levels [6]. CAI is a comprehensive term describing chronic symptoms that may arise after an acute ankle sprain, with recurrent episodes being a hallmark feature [7]. However, the effects of repetitive ligamentous and neural injury and the cumulative experience of pain associated with LAS on cutaneous sensory sensitivity of the foot remain unclear. Patients with CAI exhibit deficits across multiple domains of somatosensory function. Active and passive joint position sense at the ankle is impaired in both the frontal and sagittal planes, with greater proprioceptive errors in CAI groups than in controls [8,9]. Even in the absence of overt muscle or tendon injury, individuals with CAI demonstrate reduced ability to perceive and regulate muscular contraction during ankle movements [10,11]. By contrast, relatively few studies have examined CAI from the perspective of cutaneous sensory function. Prior work has shown diminished tactile and vibration sensation at the heel, the base of the fifth metatarsal, and the head of the first metatarsal in CAI compared with healthy controls [12,13]. Zhang, et al. [14] reported significantly higher Current Perception Thresholds (CPT) in the ATFL region (superficial peroneal nerve, L5 dermatome) in CAI patients versus controls, whereas pressure pain thresholds (PPT) measured over the common peroneal nerve trunk were significantly lower in the CAI group [15,16].
To our knowledge, no study has quantitatively assessed sensory thresholds at the ATFL and other lower-limb sites in individuals with recurrent LAS—who may be in a transitional phase toward CAI—using Quantitative Sensory Testing (QST). Accordingly, this study aimed to evaluate QST in individuals with recurrent inversion ankle sprains. Using the PainVision system (NIPRO, PS-2100), we measured CPT at the ATFL region (superficial peroneal nerve territory) and the Tibialis Anterior (TA) region (lateral sural cutaneous nerve territory). PPT was measured at the same sites using a handheld pressure algometer (TRY ALL, neutone TAM-Z2). CPT testing quantifies the function of myelinated and unmyelinated sensory nerve fibers by determining the minimum painless electrical current required to elicit sensation, thereby indexing sensory nerve activation thresholds [17]. Large myelinated Aβ fibers respond maximally to 2000 Hz stimulation, small myelinated Aδ fibers to 250 Hz, and unmyelinated C fibers to 5 Hz [18,19]. Aβ fibers transmit vibration and touch; Aδ fibers mediate mechanical nociception (e.g., pressure pain) and are primarily engaged during the acute inflammatory phase; C fibers convey dull, burning, and persistent pain and are deeply involved in post-inflammatory pain persistence and chronic pain processes [20]. PPT testing assesses deep pressure pain thresholds with a pressure algometer.
A 1-cm-diameter plastic circular probe is applied perpendicularly (90°) to the target area, and the device displays the applied pressure. When standardized protocols are used, PPT provides valuable information on the functional state of the somatosensory system [21]. The method also demonstrates high inter-rater and test–retest reliability, with Intraclass Correlation Coefficients (ICC) reported at 0.90–0.95 [22,23].
Eighteen female basketball players competing in a first-division university league participated in this study (age: 19.7 ± 1.1 years; height: 166.9 ± 5.5 cm; body mass: 61.5 ± 4.9 kg). Participants were screened and allocated into one of two groups: bilateral healthy controls or bilateral recurrent lateral ankle sprains. To exclude individuals with Chronic Ankle Instability (CAI), a questionnaire based on the guidelines of the International Ankle Consortium [24] was administered.
Eligibility Criteria
Participants were assigned to either the healthy control group or the Recurrent Lateral Ankle Sprain (RLAS) group according to the following criteria. Healthy controls. Individuals were eligible if they had no lifetime history of ankle sprain or perceived ankle instability in either limb. Recurrent lateral ankle sprain (RLAS). Individuals were eligible if they met all of the following:
• History of ≥2 lateral ankle sprains to the same ankle.
• The initial ankle sprain occurred ≥12 months prior to study enrollment.
• The index injury involved inflammatory signs (e.g., pain and swelling).
• The index injury resulted in interruption of physical activity for at least 1 day.
• The most recent sprain occurred ≥3 months prior to enrollment. • No episodes of “giving way” within the past 6 months.
• Cumberland Ankle Instability Tool (CAIT) score ≥25, confirming the absence of chronic ankle instability.
These criteria were designed to include individuals with recurrent sprains while excluding those with chronic ankle instability. This case–control study included 11 athletes with recurrent lateral ankle sprain (22 ankles) and 6 athletes without any history of ankle sprain (12 ankles), identified through screening.
Current Perception Threshold (CPT) was measured using the Pain Vision device (NIPRO, PS-2100). Pressure pain threshold (PPT) was assessed with a handheld algometer (TRY ALL, neutone TAM-Z2). The primary objective was to compare CPT and PPT between ankles with recurrent inversion sprains and ankles without a sprain history at two sites:
• Anterior Talofibular Ligament (ATFL) region—superficial fibular nerve territory; sinus tarsi: defined as the midpoint of the skin over the bony landmark immediately inferior to the ATFL.
• Tibialis anterior region—lateral sural cutaneous nerve territory; located 5 cm distal and 3 cm lateral to the tibial tuberosity.
Exclusion Criteria (common to both groups)
Participants were excluded if any of the following applied: • History of surgery, fracture, or acute traumatic injury involving the same limb.
• Systemic or dermatologic conditions that could affect foot– ankle sensation or function (e.g., diabetes, skin disease).
Chronic ankle instability (CAI), defined as meeting all of the following:
• The initial sprain occurred ≥12 months prior to enrollment;
• Inflammatory signs at injury (e.g., pain, swelling);
• Interruption of physical activity for ≥1 day at the time of injury;
• The most recent sprain occurred ≥3 months prior to enrollment;
• ≥2 episodes of giving way within the 6 months preceding enrollment; and
• Cumberland Ankle Instability Tool (CAIT) score ≤24.
All site identification and measurements were performed by a licensed physical therapist with over 10 years of clinical experience. To minimize distraction and ensure participant concentration, testing was conducted individually in a private room.
Current Perception Threshold (CPT) Assessment
CPT was assessed using the PainVision system (NIPRO, PS-2100; frequency 50 Hz, pulse width 0.3 ms). Measurements were obtained bilaterally at the Anterior Talofibular Ligament (ATFL) region (superficial fibular nerve territory) and the tibialis anterior region (lateral sural cutaneous nerve territory). Each site was tested twice, and the mean value (μA) was used for analysis. Electrical stimulation was delivered via disposable surface electrodes placed at the predefined sites. The device provided a randomized current ramp, increasing up to 124 μA over 50 s. Participants were instructed to press a handheld button with their dominant hand at the moment they first perceived the stimulus. The CPT value was defined as the minimal perceived current intensity [25]. To avoid wind-up effects, a 2-minute interval was imposed after completing bilateral measurements for each site.
Pressure Pain Threshold (PPT) Assessment
PPT was measured with a handheld algometer (TRY ALL, neutone TAM-Z2) equipped with a 1.0 cm² circular probe. The probe was applied perpendicularly (90°) to the skin and pressure was increased gradually from 0 kg up to a maximum of 9.9 kg to ensure skin safety [26]. Care was taken to prevent probe slippage, maintain a strictly perpendicular application, and control the rate of pressure increase with two-hand support while the examiner stabilized their posture. Measurements were obtained at the ATFL region and the tibialis anterior region. Participants were instructed to press the device button at the moment the sensation was first perceived as pain. For each target site on both limbs, PPT was measured three times, and the mean value was used for analysis. To minimize wind-up effects, a 2-minute interval was imposed after completing bilateral measurements for each site. We compared outcomes between ankles with recurrent lateral ankle sprain (RLAS group) and healthy controls without a sprain history (non-LAS group). The outcome measures were the Current Perception Threshold (CPT, μA) and the pressure pain threshold (PPT, kPa) at each test site. PPT values were initially measured in kilograms using a circular probe with a 1.0 cm diameter, and then converted to Kilopascals (kPa) using the following formula:
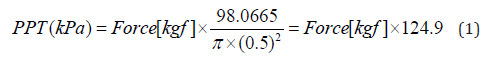
Because the distributions of these variables might deviate from normality, we employed the nonparametric Mann–Whitney U test to evaluate between-group differences. The significance level was set at p < 0.05. All procedures conformed to the Declaration of Helsinki, and written informed consent was obtained from all participants. The study protocol was approved by the Fukuoka University Institutional Review Board (approval No. K24-10-003).
We conducted Quantitative Sensory Testing (QST) of the Anterior Talofibular Ligament (ATFL) region and the tibialis anterior region in 11 athletes with bilateral recurrent lateral ankle sprains (RLAS; 22 ankles) and 6 athletes without a history of ankle sprain (non-LAS; 12 ankles) competing in a first-division university league. Outcomes included current perception threshold (CPT, μA) and pressure pain threshold (PPT, kPa). Between-group comparisons were performed for RLAS versus non-LAS ankles.
Group Means
non-LAS: CPT—ATFL 11.55±3.13μA (Median11.40μA[ 7.00–18.25μA])
TA 12.58±2.78μA (Median12.52μA[7.50–16.25μA])
PPT—ATFL 440.33±142.23 kPa (Median412.06 kPa [253.92– 727.62kpa])
TA 459.02±77.23 kPa (Median443.32 kPa [328.83–623.95kpa])
RLAS: CPT—ATFL 19.38±6.5μA (Median20.05μA [9.15– 35.50μA])
TA 12.87±2.71μA(Median12.48μA[7.65–20.30μA])
PPT—ATFL 715.47±213.02 kPa (Median709.19 kPa [349.41– 1235.42kpa])
TA 458.21±76.37 kPa (Median455.77 kPa [324.45–623.95kpa])
Between-Group Differences
At the ATFL region, both CPT and PPT were significantly higher in the RLAS group than in the non-LAS group (PPT≦p ≦ 0.01 CPT: p≦0.01) (Figures 1 & 2).
At the tibialis anterior region, no significant differences were observed between groups for either CPT or PPT (Figures 1 & 2).
Subgroup Analysis by Number of Sprains (RLAS ankles only)
Among RLAS ankles, 8 ankles had 2–4 prior sprains and 14 ankles had ≥5 sprains.
2–4 sprains: ATFL CPT 15.10±4.63 μA; ATFL PPT 619.27±167.91 kPa.
≥5 sprains: ATFL CPT 21.83±6.14 μA; ATFL PPT 770.43±216.57 kPa.
Compared with the 2–4 sprain subgroup, the ≥5 sprain subgroup exhibited a higher ATFL CPT, showing a trend toward elevation( p<0.05), whereas ATFL PPT did not differ significantly (Figure3).
Chronic ankle instability (CAI) resulting from recurrent lateral ankle sprains (RLAS) represents a multifactorial condition encompassing not only mechanical but also functional instability. To distinguish athletes who maintain ankle stability from those who experience repeated sprains over time, it is essential to understand their differences comprehensively from a sensory–neurophysiological perspective. Evidence regarding superficial sensory function after RLAS remains limited, and the influence of ligamentous and neural injury, as well as pain experience, on cutaneous sensory sensitivity in the foot has not been fully elucidated. Moreover, no prior study has simultaneously evaluated local (Anterior Talofibular Ligament [ATFL]) and distal (Tibialis Anterior [TA]) sensory function within a homogeneous athletic population. In the present study, we examined athletes belonging to the same competitive sport to evaluate the sensory function of the ATFL region and the TA region in RLAS and non-LAS limbs using Quantitative Sensory Testing (QST), specifically Current Perception Threshold (CPT) and pressure pain threshold (PPT). The results demonstrated that the CPT at the ATFL region (superficial peroneal nerve territory) was significantly higher in the RLAS group (19.38±6.5 μA) than in the non-RLAS group (11.55±3.13 μA), whereas no significant difference was found at the TA region (lateral sural cutaneous nerve territory; RLAS: 12.87±2.71 μA , non-RLAS: 12.58±2.78 μA ).
These findings indicate that sensory decline in the RLAS group is localized around the superficial peroneal nerve near the ATFL and does not extend to the distal musculature. This localized reduction in cutaneous sensitivity is unlikely to reflect innate sensory traits; rather, it suggests mechanical damage to cutaneous mechanoreceptors and sensory pathways caused by the repetitive traumatic mechanisms of lateral ankle sprains. Previous studies in CAI populations have similarly reported elevated CPTs in the ATFL region of the affected limb compared with the uninjured limb [15], supporting the current results. Burcal et al. further observed elevated Semmes–Wein stein monofilament thresholds (i.e., reduced light-touch sensitivity) in the sinus tarsi of CAI and coper groups compared with healthy controls, implying that RLAS may exacerbate superficial sensory desensitization along the lateral foot [27]. Evidence of neural injury is also substantial: surface electromyography–based conduction studies have identified mild peroneal neuropathy in 17% of grade II and 86% of grade III acute ankle sprains [28], while Falciglia et al. reported entrapment and traction of the distal Superficial Peroneal Nerve (SPN) branches after severe sprain [29]. Cadaveric biomechanical experiments have shown that SPN strain increases significantly when the ATFL is transected compared with when it is intact [30], suggesting that ATFL insufficiency may impose additional mechanical stress on the SPN in both severe LAS and RLAS cases.
In this study, a comparison within the RLAS group revealed a progressive elevation of CPT values at the ATFL region according to the number of previous sprains (15.10±4.63 in the 2–4 sprain group and 21.83±6.14 μA in the ≥5 sprain group). This dose-dependent relationship suggests that repeated sprains contribute cumulatively to sensory deterioration, potentially involving dysfunction of small-diameter myelinated (Aδ, C) and/or large-diameter myelinated (Aβ) fibers. The absence of significant differences at the TA region supports the interpretation that neural injury predominates locally within the SPN territory adjacent to the ATFL, reflecting site-specific deafferentation rather than congenital sensory variation. Regarding PPT, at the Anterior Talofibular Ligament (ATFL) region (superficial peroneal nerve territory), the RLAS group exhibited significantly higher Pressure Pain Thresholds (PPT) than the non-RLAS group (RLAS: 715.47±213.02kpa, non-LAS:440.33±142.23kPa, p < 0.01), whereas no significant difference was observed at the Tibialis Anterior (TA) region ( RLAS: 458.21±76.37 kPa, non-RLAS: 459.02±77.23kPa). Reference data from healthy Japanese females [31] indicate normative PPT ranges of 115–620 kPa (median 336 kPa) at the ATFL region and 169– 831 kPa (median 379 kPa) at the TA region. Notably, 63% of RLAS ankles exceeded the upper limit of the reference range at the ATFL region, compared with only 4.5% in the non-RLAS group, whereas no deviations were observed at the TA region in either group.
These findings collectively indicate that RLAS is characterized by elevated mechanical pain thresholds at the ligamentous region, reflecting a localized reduction in nociceptive sensitivity. Previous research has shown that acute lateral ankle sprains are associated with decreased PPT (i.e., local hyperalgesia) at the ATFL region due to inflammation and peripheral sensitization [32]. In contrast, the elevated PPTs observed in the present RLAS cohort suggest a remodeling of nociceptive thresholds through repeated injury and tissue repair. One plausible mechanism involves fibrotic and hypertrophic changes within the ligament following recurrent sprains, which may reduce the density and responsiveness of mechanoreceptors. Mechanical pain thresholds are influenced by structural factors such as free nerve ending density and tissue thickness [33]. Polymodal nociceptors— particularly free endings of C and Aδ fibers—have been identified in ligamentous and articular tissues [34], and post-injury degeneration or aberrant reinnervation of these receptors has been documented. Similarly, Sha et al. reported a decline and degeneration of mechanoreceptors within residual ACL tissue over time after rupture [35], supporting the concept of progressive sensory degradation. Such structural remodeling, combined with increased tissue stiffness secondary to fibrosis, may collectively contribute to elevated mechanical thresholds.
Beyond structural alterations, central and peripheral adaptations following repetitive injuries may also recalibrate sensory processing. Athletes with RLAS frequently resume play prematurely and may suppress pain responses to continue training and competition. This behavioral adaptation, characterized by learned pain suppression and altered threat appraisal, could lead to attenuated pain perception. Furthermore, elite athletes are known to exhibit greater pain tolerance [36], and individuals with a history of recurrent injuries demonstrate diminished subjective pain ratings to equivalent nociceptive stimuli [37]. These findings imply that, in addition to neural adaptations, cognitive and affective modulation of pain contributes to elevated thresholds in RLAS. This interpretation is consistent with Melzack’s neuromatrix theory [38,39], which posits that pain is not a direct product of peripheral input alone but an integrative output of a widely distributed neural network within the brain. Repeated injury and pain experiences in RLAS may remodel this neuromatrix, thereby altering both the threshold and the contextual meaning of pain. Such neurocognitive adaptation may represent a form of neural habituation, wherein recurrent nociceptive exposure leads to desensitization of pain perception.
Taken together, the elevated superficial sensory thresholds observed in RLAS likely arise from a multifactorial interplay of peripheral nerve injury, loss of polymodal receptors due to ligamentous fibrosis, neuroplastic reorganization within the central nervous system, neuromatrix remodeling, and psychological adaptation. These combined mechanisms may diminish pain sensitivity and blunt protective pain responses, thereby increasing the risk of recurrent injury. Therefore, comprehensive assessment of RLAS athletes should not rely solely on subjective pain reporting but should incorporate quantitative sensory testing alongside neurophysiological and psychological evaluation to capture the full spectrum of sensorimotor and perceptual alterations associated with recurrent ankle sprains.
This study demonstrated that athletes with Recurrent Lateral Ankle Sprains (RLAS) exhibit localized alterations in superficial sensory perception and mechanical pain thresholds around the Anterior Talofibular Ligament (ATFL) region. These findings indicate that pain perception in RLAS is not merely a subjective symptom but reflects complex neuromechanical and neurophysiological adaptations resulting from repeated ligamentous and neural injury. Therefore, clinical assessment of RLAS should not rely solely on subjective pain complaints or self-reported instability but instead incorporate objective, quantitative evaluations—such as Current Perception Threshold (CPT) and Pressure Pain Threshold (PPT)—to more precisely characterize sensory function. Such an integrated, evidence-based approach can improve diagnostic accuracy, guide individualized rehabilitation, and contribute to reducing the risk of reinjury and optimizing athletic performance.


