Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Niladri Dutta*
Received: August 28, 2025; Published: September 11, 2025
*Corresponding author: Niladri Dutta, Internal Medicine Trainee Betsi Cadwaladr University Health Board, NHS Wales, UK
DOI: 10.26717/BJSTR.2025.63.009862
Keywords: Immune Checkpoint Inhibitors (ICI); Pneumonitis; Immune-Related Adverse Events (irAEs); PD-1 Inhibitors; CTLA-4 Inhibitors; Steroid-Refractory Pneumonitis; T-Cell Mediated Autoimmunity
Immune checkpoint inhibitors (ICI) targeting PD-1, PD-L1, and CTLA-4 have transformed cancer treatment by increasing T cell–mediated anti-tumor immunity and extending survival in a variety of cancer types. However, immune activation leads to the possibility of immune-related adverse events (irAEs). Despite being uncommon, pneumonitis is a serious immune-related toxicity that can be fatal, resulting in respiratory failure, and require treatment termination. It is essential for both oncologists and pulmonologists to understand its epidemiology, pathogenesis, and treatment.
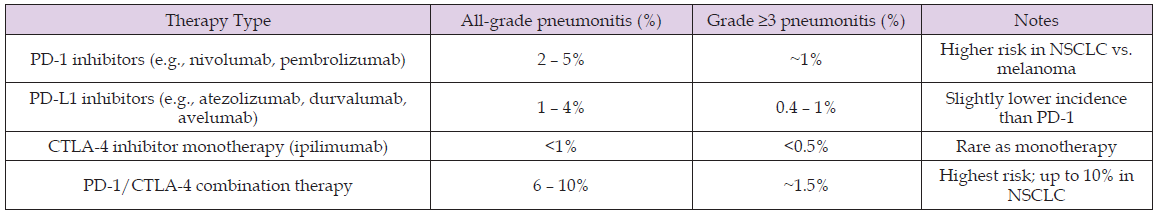
The incidence of ICI-related pneumonitis (CIP) depends on the ICI-agent and its respective regimen. PD-1 inhibitors such as nivolumab and pembrolizumab are associated with all-grade pneumonitis in ~2–5% of patients, with grade ≥3 events in ~1% [1,2]. PD-L1 inhibitors carry a slightly lower risk (1–4%), whereas CTLA-4 monotherapy rarely causes pneumonitis (<1%). Combination regimens, particularly PD-1 plus CTLA-4, have the highest risk (6–10% overall; ~1.5% severe) of CIP [2,3]. Real-world series suggest higher incidences (5– 12%), particularly among patients with lung cancer or pre-existing interstitial lung disease [4]. Additional risk factors include prior thoracic radiation, smoking history, and chronic obstructive pulmonary disease.
CIP is thought to be a result of T-cell–mediated autoimmune response to ICIs. Shared antigenic targets between tumor cells and type II pneumocytes, and overlapping T-cell receptor clonotypes in tumor and lung tissue, support this hypothesis [5,6]. Histology frequently shows cryptogenic organizing pneumonia (COP), though Non-Specific Interstitial Pneumonia (NSIP), hypersensitivity pneumonitis, diffuse alveolar damage, and granulomatous inflammation are also reported [7]. Cytokine analyses demonstrate elevated IL-6, IL-17A, and granzyme A in severe cases, implicating dysregulated immune signaling [8,9]. Additional contributors may include anti-CD74 autoantibodies, microbiome modulation, and genetic susceptibility (e.g., HLA alleles) [10]. The heterogeneity of radiologic and histologic patterns suggests converging immunopathogenic pathways.
CIP typically arises weeks to months after ICI initiation but can present late. Symptoms include cough, dyspnea, and fever, though some patients remain asymptomatic (Table 1). HRCT Thorax reveals patterns such as COP, NSIP, hypersensitivity pneumonitis, or ARDS-like diffuse alveolar damage [7]. Diagnosis is clinical and relies on excluding infection, tumor progression, and radiation pneumonitis. Recommended workup includes HRCT, bronchoalveolar lavage, and, where necessary, tissue biopsy. Multidisciplinary input is strongly recommended.
Table 1: Reported incidence of immune checkpoint inhibitor–related pneumonitis, stratified by therapy type and severity (all-grade vs. grade ≥3).
Management is guided by CTCAE severity: [1,3]
• Grade 1 (asymptomatic): Withhold ICIs, monitor; approximately
30–40% resolve without steroids.
• Grade 2 (symptomatic): Discontinue ICIs; initiate oral
prednisone 1–2 mg/kg/day. Most respond within 1–2 weeks.
• Grade ≥3 (severe/life-threatening): Hospitalize; treat
with IV methylprednisolone 1–2 mg/kg/day, taper over ≥4–6
weeks. Up to 30% are steroid-refractory.
• Steroid-refractory: Consider infliximab, mycophenolate, or
IVIG; ~50–60% achieve improvement. [4,5].
Mortality in high-grade CIP ranges from 10–20% [6]. Permanent
ICI discontinuation is common in grade ≥2 pneumonitis (~80%). Rechallenge
may be attempted after full resolution in selected patients,
with success rates of 60–70% but recurrence in ~25% [7].
Current gaps include the absence of standardized diagnostic criteria, reliable biomarkers, and evidence-based strategies for steroid- refractory CIP. Long-term pulmonary sequelae remain poorly characterized. Advances in radiomics, AI-based imaging, circulating biomarkers, and large-scale registries may enable earlier detection, improved risk stratification, and targeted therapies.
ICI-related pneumonitis is an uncommon but serious complication of cancer immunotherapy. Early recognition, multidisciplinary evaluation, and timely corticosteroid initiation are critical. Improved biomarkers and therapeutic strategies for refractory disease are urgently needed. Ongoing translational research will be key to optimizing outcomes while preserving the benefits of immunotherapy.


