Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Anup Kumar Sarkar*
Received: August 23, 2025; Published: September 09, 2025
*Corresponding author: Anup Kumar Sarkar, Department of Botany, West Bengal State University, Berunanpukuria, Malikapur, Barasat, Kolkata-700126, West Bengal, India
DOI: 10.26717/BJSTR.2025.63.009856
The eggs and different instars of domesticated Eri silkworm (Philosamia recini Hutt.) were irradiated with 40 Gy (4000 rads) of 60Co gamma rays. The 2, 4, 6, 8, and 10 days old of 5th instar larvae exposed to gamma rays and significantly shows that hatchability and moulting period are longer than in the control. The nature of the Synaptonemal Complex (SC) aberration (ring chromosome and fragmentation) subsequently increases from day 2 to day 8. From day 10 the frequencies of aberration become less. The overall observation showed the mutagenic and teratogenic activity of gamma rays on gonadal cells.
Keywords: Eri Silkworm; 60Co; Gamma Rays; Hatchability; Synaptonemal Complex
Meiosis is a complex process, generally consisting of two divisions and stages /substages. Essential to this process is the pairing of homologous chromosomes into a bivalent, often which recombination of genetic material occurs. The pairing and segregation of the chromosomes is mediated by the tripartite proteinaceous structure called the Synaptonemal Complex (SC) [1,2], which is highly conserved through evolution [3]. SCs are normally found in all sexually reproductive organisms. Some exceptional cases are their males of Drosophila ananassae [4]. Synapsis of homologous bivalents i.e., SCs leads to crossing over [5], no crossing over occurs normally in the silkworm female [6]. In several cases of a chiasmatic meiosis, the biogenesis and the function of the SC differ from those of chiasmatic meiosis. At the diplotene, short stretches of SCs are retained at the position of chiasmata. [7-12]. The SCs in surface spreading and silver stain technique for the quantitative and qualitative analysis have advantages of accuracy, simplicity and sensitivity and rapidity [2, 8,11]. The alkylating agent mitomycin C (MC) slightly but significantly induces much more interchange type recombinants than exchange type ones in early growth stage of the 5th instar silkworm larvae [13]. MC and cyclophosphamide cause meiotic toxicity [14] in mouse spermatocytes. Acridine orange and cycloheximide causes meiotic abnormalities in Candida albicans [15]. The antibiotic like puromycin, actinomycin D, daunomycin causes meiotic alterations and germ cell damages [16-18].
Cycloheximide causes meiotic alteration of SC [19] in vitro in Lily meiocytes. [20] reported its effect on chiasma frequency reduction in Lily. Reduction of chiasma frequency also reported by [20-23]. Radiation, the physical mutagen, causes damage to the eukaryotic and prokaryotic genetic materials. X-rays induce the hereditary change in the mice [24] by the alternating arrangement of chromosomal aberration in the pre-spermatocytes cells of different system causes by ionization radiation viz. in human [25], barley [26], grasshopper [27], mice [28-30], Philosamia ricini [31]. In vitro condition shows the division stages i.e. M-phase is more prone to chromosomal damage by irradiation [32]. 60Co gamma rays alter the synaptic behaviour and SC damages [33]. It means that irradiation causes direct or indirect damage to disturb the mitotic cell divisions [34] and leads to chromosomal aberrations [35]. The quantitative analysis of SCs damage reported on Syrian hamster [36]. The pachytene cells are radiosensitive than prepachytene stages [37]. Sensitivity of gamma irradiation varies in different stages of development of house fly (Musca domestica) in pupa stage [38] and the fruit beetle [39]. It also proposed that Trogoderma granarium insect larval stages are highly radio-resistant [40]. On the other hand, the domesticated silkworm Philosamia ricini male pupaspermatocytes show that chiasma frequency significantly increases in lower doses and decrease in higher doses [31]. In general, it also reported that young one is more sensitive than the older one in larvae stages [41].
So far very little information is available as to 60Co gamma rays’ radiation induced mutagenesis in meiotically dividing cells particularly on SCs of silkworm (Philosamia ricini) using surface spread and silver staining technique. The present investigation analysis the SCs of the post gamma irradiation silkworm with 40 Gy in different days at different larval stages. The results indicate the formations of ring chromosome and fragmentation of SCs.
Eggs of Philosamia ricini Hutt. (Lepidoptera, Saturniidae, 2n=28) the domesticated silkworm was collected from the Manipur Govt. Farm, Goyaltabi (Latitude 24.91906° or 24° 55′ 9″ north and Longitude 94.13658° or 94° 8′ 12″ east), Manipur, India, in 1989. Larvae were reared in the Laboratory of Life Science department of Manipur University, Canchipur, Imphal, Manipur, India, at room temperature ranging from 15°-19° C. They were fed with castor (Ricinus communis) leaves. The eggs and worms (larvae) were irradiated by 60Co Gamma rays with a dose rate of 1.108 Gy/second at the radiation centre of Manipur University. The eggs were irradiated with 40 Gy radiation to see its effect on hatchability and life cycle. The same dose was given to the group of silk worm larvae at day 1st of 5th instar to evaluate the Synaptonemal Complexes (SC). A control group of similar age of l arvae was also maintained for comparative analysis. The surface spread and silver stain method of Fletcher [42], which is modified by Bhagirath, et al. [43] was used for preparation of Synaptonemal Complexes (SCs).
The Detailed Procedure is as Follows
Preparation of Cell Suspensions: The worms were killed by the pressure on the head in different days of the 5th instar. The worms were dissected under the zoom stereo microscope (Meiji EMZ-2). The gonads were removed and placed in Eagle’s Minimum Essential Medium (MEM) at room temperature (15°- 19°C). The fat bodies were removed from the gonads with the help of tungsten needles. The precaution was taken not to release the cells from the gonads. After removal of all unwanted materials, the gonads were punctured with a tungsten needle in the MEM solution.
Preparation of Solutions:
a. Fixatives: The 4.0gm of paraformaldehyde (Sigma Chemical Company, Lot No. 17 F- 0605, St. Louis No. 63178 USA) was added to distilled water to make up to 100 ml. The suspension was stirred with a magnetic stirrer, maintaining the temperature at 60° C for at least 30 minutes then added 6 drops of 1N NaOH. A pinch of phenol red was put before adding 1N NaOH to get light colour. The solution was allowed to cool down at room temperature and adjusted the pH 8.2 by using borate buffer. It was filtered before use.
b. Washing Solution: 0.4 % solution of wetting agent (Kodak Photoflo) [44] was made fresh and filtered before use. The pH was adjusted at 8.2 with borate buffer.
c. Staining solution: 70% (W/V) of AgNO3 (Sigma Chemical company) was made in distilled water. The solution was filtered through 0.22μ (Swinnex) Millipore filter into a brown dropping bottle. It was kept in a refrigerator in the dark chamber for use.
Preparation For Spreading Cells:
a. Micropipettes were prepared from capillary tube and attached to the mouth piece of polyethylene tube.
b. Using the pointed micropipette one drop of cell suspension was made to fall from a height of 2 mm directly on the surface of the slide containing 2 large drops of 0.5 % NaCl solution and allowed to spread cells properly 30-40 second.
c. The cells were then picked up on a marked clean slide (First soapy water, then distilled water, and lastly alcohol) by touching the surface of the spreading solution. The slides were left flat to settle down the cells on the slide tray. Precaution was to keep the tray at horizontal level.
d. The slides were then fixed in the fixative (4% paraformaldehyde prepared as above) on vertical couple jar for 7 minutes. After draining the excess fixative, the slides were washed in 0.4% wetting agent solution for 20-30 seconds. After every 5 slides the wetting agent solution was discarded owing to accumulation of fixative in the solution. The slides were then allowed to dry in air at room temperature in a dust free condition.
e. Staining of the slides were performed by placing 3 drops of AgNo3 (70% w/v) solution on the slide and a cover glass (size 24 x 60 mm) was placed on the top. The slides were kept for 72 hours a water bath, maintaining a temperature of 55° C. The cover glass was removed and slides were washed several times in a vertical coupling jar with distilled water and air dried.
f. The air-dried slides were scanned under the compound microscope first at low power. If the good spread with well stained cells were found then scanned under higher magnification. Selected pachytene spread cells were scanned under oil immersion for SC analysis and other synaptic irregularities.
g. Microphotography: The selected cells were photographed by using 25ASA ORWO film in a Leitz-Diallux 22 fitted with Vario-Ortho mat camera.
The eggs and different instars of silkworm were irradiated with 40 Gy 60Co gamma rays. After the 4th moulting of the 5th instar larvae were used for the dissection of the gonads. The gonads of the Eri silkworm (Philosamia ricini) were white and paired. They were present at the 8th abdominal segment of the dorsal part of the body. From the starting of 5th instar, the pachytene cells increased to a high frequency and continued up to the end of this instar. It was reported that pachytene cells are more radioactive than pre-leptotene/spermatogonia cells [37]. The synaptonemal complex (SC) preparation on different days irradiated 5th instar larvae (day 2, 4, 6, 8, and 10) was performed by using surface spreading and the silver-stained technique.
The Moulting Period and Abnormalities Observed in Different Days of Irradiated 5th Instar Larvae were Studied under the Following Conditions:
Eggs: Five days after laying, the eggs were irradiated. All control eggs hatched within 16 days, whereas the irradiated eggs hatched after 25 days (Figure 1).
1st Instar: The voracious feeding, strong and healthy larvae were irradiated, but after one day of irradiation, the larvae were looking dull and took less amount of castor leaves, whereas the control larvae were voraciously feeding and were larger in size than the irradiated larvae. Comparatively moulting period was long in the irradiated larvae (7 days) than the control larvae (5 days), as shown in Figure 1. In this instar the behaviour was distinctly remarkable; the control larvae showed moving easily but irradiated larvae were slow.
2nd Instar: Similarly, irradiated 2nd instar larvae showed weakness from day one and onwards and were feeding very small amount of castor leaf. Not much difference was seen between the 1st instar and 2nd instar just after two days and onwards. Comparison with the irradiated 2nd instar and the control 2nd instar showed distinct variable lengths. The control larvae were larger than the irradiated ones. Moulting period for irradiated and control larvae was 5 days and 4 days, respectively.
3rd Instar: There were no distinctly behavioural differences between the control and irradiated 3rd larvae except for the moulting period. In case of control larvae, the moulting took place within 6 days, whereas irradiated larvae required 8 days (Figure 1). Along with other physiological characters, such as blue or white colour was observed in both cases, and secretion was also not distinctly different.
4th Instar: There were no distinguishing characteristics exist between the irradiated and control larvae. Only some larvae were somewhat smaller in size than the control larvae. Moulting period for the irradiated larvae and control larvae was 7 days and 5 days, respectively.
Effect of 60Co Gamma Rays on Different Days of the 5th Instar Larvae of Philosamia ricini
Day 2 of 5th Instar: The irradiated day 2 of the 5th instar larvae behaved similarly to the 4th instar larvae. Both control and exposed larvae were feeding on castor leaves in quantitatively same amount but only the duration of the moulting period of the 5th instar larvae showed little differences. In the case of irradiated and control larvae, the moulting period occurs 7 days and 6 days, respectively. The spermatocytes and Oocytes showed the minimum damage in SCs (Table 1) of the pachytene stage of meiotic prophase-I. The chromosome with a ring and the fragmentation were distinctly visible (Figures 2-4). The ring chromosome frequency was lowest, followed by fragmentation. The total aberrant pachytene cells were 19 (17.11%) (Table 1).
Table 1: The structural abnormalities of SCs of irradiated silkworm larvae at different days interval of 5th instar with 60Co gamma rays (40 Gy).
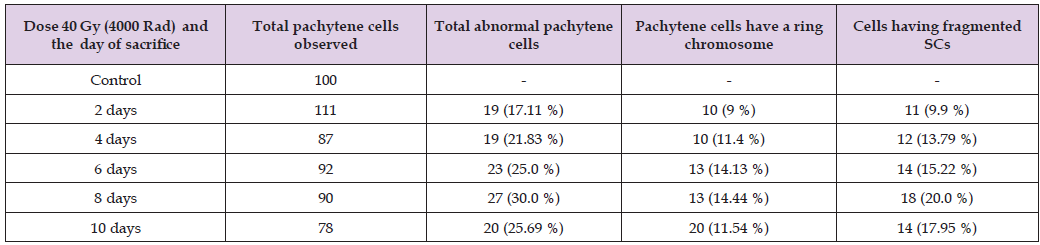
Note: ( ) shows the frequency.
Day 4 of 5th Instar Larvae: The germinal cells of the irradiated day 4 of the 5th instar showed somewhat different results than the irradiated day 2 of the 5th instar. The frequency of Aberrant pachytene cells was increased from 17.11% to 21.83% (Table 1). The frequency of aberrant pachytene cells having ring chromosomes and fragmented SCs were 11.4 % and 13.79 % respectively.
Day 6 of 5th Instar Larvae: In the study of larval behaviour, it was observed that the irradiated larvae looked similar to the control larvae. They were feeding voraciously and were healthy and strong. The frequency of aberrant pachytene cells 25 % consisted of fragmented SC and ring chromosome with the frequency of 15.22% and 14.13 % respectively (Table 1).
Day 8 of 5th Instar Larvae: The day 8th of the 5th instar behaved just like the control larvae. The amount of leaves consumption was also similar to the control. Day 8th of 5th instar germinal cells showed the maximum damage of SCs (30.0%). The fragmented and ring chromosomes were distinctly visible with the frequency of 20.00 % and 14.44 % respectively (Table 1). The day of 8th of the 5th instar larvae showed the maximum frequency of fragmentation of SCs, i.e., 20.00% (Table 1 & Figure 4).
Day 10 of 5th Instar Larvae: The irradiated day 10 of the 5th instar larvae looked somewhat dull. Probably, they were just preparing themselves to form a cocoon. The consumption of food was a smaller amount. The abnormal pachytene cells were found lesser than the 8th day of the 5th instar larvae. The frequency of the aberrant pachytene cells was 25.69 % (Table 1). The frequency of the ring chromosome and fragmented SCs (Figures 2 & 3) was 11.54 % and 17.95 % respectively.
The estimation of 60Co gamma rays’ effect on hatchability of eggs with 40 Gy resulted significant delay in hatching and subsequent moulting period. The eggs after 5 days of oviposition were used for irradiation. Analysis of synaptonemal complexes (SCs) of the 5th instar irradiated larvae revealed the genetic hazards to the silkworms. The gamma rays’ effects on the economically important silkworm system in vivo were evaluated for their mutagenic activities as follows,
• The 40 Gy of gamma rays causes delay in hatching and subsequent
moulting periods.
• The sublethal dose i.e. 40 Gy causes fragmentation of SCs.
• Radiation also causes the formation of ring chromosomes.
Effect on Hatchability and Moulting
The investigation shows the effect of 40 Gy gamma on eggs after 5 days oviposition delay in hatching for 9 days. The control eggs hatch in 16 days whereas the irradiated eggs hatched in 25 days. Tazima (45) showed that LD50 for 7 days oviposited eggs was 65 Gy and also caused a delay in hatching and moulting. The above experiment with 40 Gy irradiation at 5 days old eggs showed delay in hatching but all the eggs were hatched. The gamma rays’ effect on 5-day-old eggs does not show much genetic hazards. The duration of moulting of different instars increased comparing to the control groups. This may be due to slow physiological process or radiation may hamper the hormonal regulation. No larvae died during their life cycles. Silkworms are generally radio sensitive in the early stages of life cycle and larvae are more radio resistant than eggs. Pupae and adults are extremely resistance to radiation [45].
Fragmentation of SCs
The effect of gamma rays on SCs with the dose of 40 Gy which caused the delay in hatching and moulting revealed extensive damages. The insects were irradiated with 40 Gy at day 1 of the 5th instar larvae. SCs were prepared on days 2, 4, 6, 8, and 10 of post-irradiation. The results of different days showed the fragmentation of SCs though the frequency were different. The fragmentation of frequencies were increased up to day 8 at the peak point and decreased at day 10. The fragmentation of SCs after radiation normally occurs due to the blockage of DNA synthesis, which is a repair type [37]. It is also reported that fragmentation of chromosome is induced by X-rays in Bombyx mori [6]. The present experiment shows analogous results with 40 Gy gamma rays. The frequencies of fragmentation reduced at 10 of post irradiation way due to new cell generation or degeneration of abnormal cells. The aberrations of the meiotic chromosomes of Tetranychus urticae Koch have also been observed in X-irradiated gonadal cells [46].
Formation of a Ring Chromosome
The formation of a ring chromosome in the pachytene stage with 60Co gamma rays indicates the structural abnormalities. Regarding the ring chromosome formation [47] hypothesized that the agents (physical / chemical) action on the chromosome leads to the formation of raw sticky ends, and then these sticky ends adjoin due to tension, leading to the formation of the ring chromosome. In general, for monokinetic prophase-I, the ring chromosomes are observed at diakinesis of meiotic prophase-I, and they form due to translocations.
Silkworms possess a holokinetic nature of chromosomes; there is no possibility of forming translocation [46]. The ring chromosome which are seen in the present experiment may be due to the adjournment of the sticky ends. No report is available about the formation of a ring chromosome in pachytene stage. It has been observed that the dose of 40 Gy ionizing radiation causes delay in hatching and moulting, mutagenicity and teratogenicity in silkworm.
The author declares that there is no conflict of interest.
The author sincerely acknowledges the Head of the Department of Life Sciences and the In-Charge of the 60Co Gamma rays chamber, University of Manipur, Canchipur, Imphal, India, for providing all facilities to complete the research work.


