Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Raul Ionuț RITI*, Andra Emanuela PLEȘA CHIOREAN and Eliza Elena SUCIU
Received: August 19, 2025; Published: September 04, 2025
*Corresponding author: Raul Ionuț Riti, Technical University of Cluj-Napoca, Faculty of Industrial Engineering, Robotics, and Production Management 400114 Cluj, Romania
DOI: 10.26717/BJSTR.2025.63.009853
Orthodontic splints, traditionally regarded as adjunctive appliances for retention or occlusal stabilization, remain paradoxically under-theorized as biomedical devices despite their ubiquity in clinical practice. This study addresses this critical gap by reframing splints through biomedical engineering, regulatory science, and translational healthcare. Using a sequential mixed-method design, the research integrates longitudinal clinical trials, standardized laboratory assays for cytocompatibility and mechanical resistance, and expert interviews with orthodontists, biomaterial scientists, and regulatory specialists. Results demonstrate that digitally fabricated splints produced through computer-aided design, computer-aided manufacturing, and three-dimensional printing significantly outperform conventional acrylic appliances in retention stability, functional rehabilitation for bruxism and temporomandibular joint dysfunction, biocompatibility, and intraoral durability.
Patient adherence, captured through digital monitoring systems, exceeded eighty percent in digitally fabricated devices, highlighting comfort and aesthetics as biomedical determinants of therapeutic success. Beyond clinical and laboratory validation, the study advances a regulatory reframing by embedding orthodontic splints within European medical device regulation, United States food and drug governance, and international quality management standards. By bridging dentistry, biomedical engineering, and regulatory governance, this work introduces a paradigm shift that elevates orthodontic splints from clinical adjuncts to rigorously validated biomedical devices. In doing so, it resolves longstanding methodological fragmentation while charting a translational roadmap for safer, sustainable, and globally standardized orthodontic care.
Keywords: Orthodontic Splints; Biomedical Device Validation; Computer-Aided Design and Computer-Aided Manufacturing Fabrication; Regulatory Compliance Under International Standards; Translational Biomedical Research
Abbreviations: AI: Artificial Intelligence; AGI: Artificial General Intelligence; ANOVA: Analysis of Variance; CAD/ CAM: Computer-Aided Design / Computer-Aided Manufacturing; CI: Confidence Interval; EMG: Electromyography; FDA: U.S. Food and Drug Administration; GDPR: General Data Protection Regulation; HGF: Human Gingival Fibroblast; HPLC: High-Performance Liquid Chromatography; ICC: Intraclass Correlation Coefficient; ISO: International Organization for Standardization; MDR 2017/745: Medical Device Regulation (European Union, Regulation 2017/745); MTT assay: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (cell viability test); NVivo: Qualitative Data Analysis Software (version 14 used in this study); OR: Odds Ratio; T0, T1, T2: Baseline, 3-month follow-up, and 6-month follow-up time points; TMD: Temporomandibular Disorder; VAS: Visual Analogue Scale
Orthodontic splints have been acknowledged as an irreplaceable part of the orthodontic treatment, playing a significant role in the maintenance of post-treatment outcomes, occlusal stabilization, as well as management of functional disorders, like bruxism and temporal- mandibular joint dysfunction (TMD) [1-3]. Conventional systems such as the Hawley retainer and fixed lingual retainers have had ample coverage in the orthodontic literature as conventional strategies of post-treatment stabilization and reducing relapse [4-6]. Vacuum- formed thermoplastic retainers have also added diversity to clinical practice and are better aesthetically, more comfortable and patient acceptable, which is why they contribute to long-term compliance [7]. However, even in the scope of the current innovations, orthodontic splints cannot leave the subject of a seeing opportunity as something secondary to orthodontic treatment, and they are unlikely to be conceived of as the subject of regulatory oversight and testing beyond the context of an orthodontic appliance [8]. Such a shortsighted attitude restricts both academic debate and clinical action, especially with regard to long-term safety, reproducibility, and standardization. Orthodontics has gone through a decade of digital technologies and further improved biomaterials that have changed the domain. The precision and reproducibility of splints have improved with the utilization of computer-aided design/ computer-aided manufacturing in the fabrication process of splints, which provides more accurate and reproducible devices compared with one-to-one fabricated acrylic appliances [2,9].
Studies have shown CAD/CAM retainers and 3D-printed splints have a better fit and mechanical resistance than conventional acrylics [3,8]. But in the drive to extol the operational success and marginal clinical benefits of these technological innovations, one cannot claim that these technological innovations have undergone a robust biomedical appraisal. Areas that would need more research include cytocompatibility, release of leachable monomers, long-term intraoral performance, and sterilization procedures, although they are central to achieving international medical device requirements like ISO 10993, ISO 20795, and MDR 2017/745 [1,7,10]. Such disproportion of clinical functions and biomedical examination are yet more evident when orthodontic splints are placed into the wider picture of the global pattern of medical devices regulation. The Medical Device Regulation (MDR 2017/745) applies to the European Union, the United States Food and Drug Administration (FDA) of classification, and ISO 13485 standard of quality management necessitate risk management evidence, traceability, and clinical validation of intraoral devices, in cases wherein the product is in continuous contact with oral tissues [5,9]. Although dental implants, prosthetic structures, and surgical templates are already well-researched in this regulatory matter [6,10], orthodontic splints are virtually unexplored in this regulating context despite its popularity and relevance on oral and systemic health.
Not only does this lack show a gap in the scholarship but poses practical risks as the splints are not within the domain of a regulatory validation, which prevents informational interdisciplinary integration between the spheres of orthodontics, biomedical engineering, and regulatory science. In order to fill this gap, this study proposes a paradigm shift that acknowledges the biomedical nature of orthodontic splints themselves by overtly classifying splints as such. The study will be framed by three questions: first, do 3D-fabricated splints in general attain better clinical performance in retention stability and functional rehabilitation as compared to traditional acrylic appliances [11,12]; second, how do new polymers and additive fabrication processes affect biocompatibility, safety, and durability as tested in standardized ISO and MDR testing guidelines [13,14] and third, how far can orthodontic splints be integrated into the global regulatory practice with respect to reproducibility interdisciplinary With the centralization of such questions within the investigation, the research will have a systematized research agenda that will help to harmonize orthodontic scholarship and biomedical device science. The current study is three-fold in its contribution and is graphically summarized by Figure 1 that projects orthodontic splints in a biomedical device context on the axes of clinical, material, and regulatory dimensions. Theoretically, the study re-contextualizes the role of orthodontic splints, which do not come to light only in a classical context of dentistry, but are incorporated into a wider epistemological field of biomedical innovation and regulation.
Empirically, it operationalizes a mixed-method approach that triangulates longitudinal clinical trials, standardized in-vitro assays of cytocompatibility and mechanical durability, and qualitative expert validation and hence both methodological rigor and translational breadth. In practice, it echoes a systematic integration route plan, by aligning orthodontic splints with the ISO, MDR and FDA regulatory aspects to promote patient safety and device traceability, and sustainability of healthcare. Through the convergence of these three layers-clinical performances, biomaterial safety and systemic regulation- Figure 1 shows how orthodontic splints can be repositioned as rigorously-validated biomedical devices as opposed to discretionary orthodontic adjuncts. Collectively, these contributions provide a direct solution to the identified gaps in the orthodontic and biomedical literature and contributes in line with the journal mission statement which is to drive the development of interdisciplinary and translational discoveries in device-related healthcare [15].
Orthodontic splints have become a cornerstone in orthodontic practice, especially in retention, teeth fitting, and treatment of functional disorders of the vertical plane like bruxism or temporomandibular joint syndromes (TMD) [133. Classic appliances (such as the Hawley retainer or fixed lingual retainers) have long been discussed in the literature [4-6]. More recent regimens, including vacuum-formed thermoplastic retainers, have widened treatment possibilities by combining the highest quality and more patient palatable looks [7]. Nevertheless, due to the size of the literature on the topic of relapse prevention, stability, and patient compliance, splints have still remained mostly conceptualized as a traditional orthodontic adjunct as opposed to a biomedical device with patient regulatory consideration and standardization. Such a conceptual drawback limits the scientific and medical debate regarding the long-term safety, durability, and standardization of them. Orthodontic splints have seen tremendous progress in recent years in the understanding, design, and production through the innovations of digital workflows and new biomaterials. Computer-aided design/ computer-aided manufacturing, additive manufacturing, and pressure-molded thermoplastics have transformed not only efficiency but also led to increased precision, allowing highly customized devices with better fit and reproducibility [1,8]. Literature shows that CAD/CAM and three-dimensional (3D) printing improved adaptation and mechanical accuracy compared with conventional acrylic-based methods [2,9].
However, as much as such technological innovations are often hailed, the discussion often stays in the arena of the feasibility of use in practice and marginal benefit in the clinical setting. What is lacking in this discussion is a critical biomedical assessment of this area-including biocompatibility of the material, longevity of mechanical resistance during normal chewing forces, sterilization procedures, and conformity with existing international standards regarding the use of medical devices [2,8,10]. In addition to retention, occlusal splints are an essential stepping stone in addressing functional status, including bruxism and TMD [3,13]. Benefits related to reduction in muscular hyperactivity as well as pain and functional outcomes are reported in most systemic reviews [11,15]. However, the empirical evidence is by no means conclusive. The research on this issue often contains inconsistencies in the methodology, poor sample publications, the short length of the follow-up, and the heterogeneity of definitions of devices and outcomes [15,16]. What happens is the creation of a disjointed body of evidence that stresses on clinical utility at the same time exhibiting a lack of standardized protocols. Specifically, improper taxonomy and confirmed assessment measures reduce the translational value of studies, exposing clinicians and patients to inconsistency in the quality of devices and treatment effects [11,12].
Despite the emerging use of technologies and high-performance polymers in the production of orthodontic splints, the discipline is undermined in the literature of biomedical devices [1,9]. Regulatory environments, e.g., the European Union medical device regulation (MDR 2017/745) [10] and the classification system in the United States Food and Drug Administration (FDA) [14], as well as ISO 13485 standards, contain clear statements about risk management, traceability, and clinical validation [10,14]. There has, however, been little scholarly focus on such splints in this regard, and most regulatory and quality-assurance studies have either focused on implants, surgical guides, or prosthetic frameworks [9,13]. The lack of this perspective on dental orthodontic splints is conspicuous because they are commonly used, in direct contact with the patient, and have the potential to affect oral and systemic health. Conceptualizing splints explicitly as biomedical provides, therefore, rather a paradigm shift than a semantic shift; one that supports orthodontic practice within the wider expectations of biomedical innovation and patient safety [1,13]. Collectively, the literature indicates that although orthodontic splints have a long history of clinical use in orthodontics, they have seldom been examined through the biomedical and regulatory perspectives that characterize modern medical device research [2,14].
The outcome is a paradox of practice and under-theorization: splints permeate the practice but have little theory around them. Current literature skews towards clinical usefulness and patient outcome, without incorporating a discourse on device classification, biocompatibility, sterilization and manufacturing validation [8,10]. This paucity forms the intellectual and pragmatic basis of the current study that attempts to reorient orthodontic splints back to the biomedical device paradigm. In synthesizing the existing orthodontic knowledge, including regulatory frameworks, and then demonstrating the use of that knowledge in clinical practice through detailed case studies, the paper assists in closing the gap between dentistry, biomedical engineering and health policy-a gap that is keenly needed to promote a safe, innovative, and sustainable orthodontic practice - Figure 2 [1,13].
Repositioning of orthodontic splints as biomedical devices needs a systematic sequence of clinically-based hypotheses, material science, and biomedical regulatory systems. As opposed to conventional appliances, which were characterized by research based on retention and functional therapy, the modern use of splints made in contemporary digital technologies depicts a combination of novelty, safety, and patient-centered manufacture. In order to assess such multidimensional claims, the current research formulates hypotheses in four related areas, namely clinical effectiveness, material biocompatibility, patient adherence, and systemic biomedical integration. Clinically, orthodontic retention is perhaps the most debatable issue, and the relapse often compromises the effects of treatment. Though Hawley retainers (acrylic-based) are still considered an option, the results of recent studies have demonstrated that the thermoformed and CAD/CAM splints demonstrated higher predictability and lower relapse rates [1,2]. There is parallel evidence that can prove the value of the occlusal splints in the control of bruxism and TMD, relieving the hyperactivity and muscular pain [3,4,17]. It is based on this evidence that it is expected that a splint created with the more advanced method will not only maintain the post-treatment alignment, but will additionally have a functional outcome enhancement with respect to patients with parafunctional or TMJ dysfunctions.
Accordingly, we hypothesize that H1 and H2 are associated with knowledge of the same, retention stability, and splint therapy in relation to functional rehabilitation in the case of digital fabrication. No less important is the question of material safety. Although heat cured acrylics have largely become commonplace, they continue to be linked with monomer release and inconsistent intraoral tolerance. Current new technologies in the field of polymer chemistry and 3D-printing resins have provided materials with greater cytocompatibility and lower allergic potential [5,6,18]. In addition, it is stated that the devices produced with the help of digital fabrication offer better fit, mechanical capacity, and durability than conventional thermoformed retainers [7,8,14,19-21]. The results confirm H3, advanced polymers have better biocompatibility compared to conventional acrylics, and H4, digital splints have an incremented level of mechanical resistance when compared to the intraoral load [22]. As has consistently been presented in the literature, transparent, thermoplastic splints are more accepted by the patient than the bulkier wire-based splints or acrylic-based ones are, mainly because of aesthetics and comfort [9,10,23-25]. Since compliance is a prerequisite to clinical efficacy, a digitally synthesized splint is expected to produce a greater amount of compliance and satisfaction. H5 is anchored on these discoveries that associate digital thermoplastic design with patient-reported comfort and adherence.
Lastly, the biomedical framing brings forth regulation and cost issues, as well as interdisciplinary integration. The MDR and ISO 13485 regulatory framework are meant to provide patient safety by ensuring the validated workflow, risk management, and traceability are standardized [11,12,15]. However, orthodontic splints have seldom been tested in line with such regimes, although the additional boom of the prescribing of intraoral plasters is common. Moreover, digital workflows require more up-front investment but lead to a reduction in fabrication time and material wastage, hence more cost-effective and scalable in the long-term [8,15,16]. Placement of positioning splints in the biomedical device paradigm is thus speculated to reduce the occurrence of negative consequences, increase reliability, and spur multidisciplinary cooperation of orthodontics, biomedical engineering, and regulatory science [26]. H6, H7, and H8, focus on safety, cost-efficiency, and integration in the system and cover these expectations. In this study, the following are the main hypotheses as summarized in Table 1 below.
Collectively, the eight hypotheses lead to a clear language of integration between clinical performance, biomaterial safety, patient-focused outcomes, and regulatory validation. As outlined in Table 1, each hypothesis is clearly grounded by recent evidence and up-to-date biomedical findings, which is associated with repositioning orthodontic splints toward medical-regulated products, and matches the translational and interdisciplinary nature of this journal. In addition to giving the current study an empirical basis in advancing cutting-edge literature, this comprehensive approach also serves to organize the procedural perimeter of the proposed empirical approach, available as will be explained in the next section dealing with methodology.
This study was specifically designed with methodological systematization that aimed to eliminate the disjointed, descriptive, and anachronistic protocols that have traditionally defined orthodontic splint studies. The integration of orthodontic appliances into a mixed-method approach to biomedical device validation is multidimensional in removing orthodontics to the limits of internationally accepted biomedical validation practices, and in directly responding to issues of clarity, rigor, and contribution as expressed by the reviewers. Namely, the design incorporates all three clinical, laboratory, and regulatory perspectives into a sequential theory-driven modeling that operationally transforms the eight hypotheses developed in the preliminary stages in a coherent manner. This will ensure the lack of arbitrariness in the investigation, but in the logic of developing the hypotheses, collecting data, and triangulating the analyses [1,11,21]. Fundamentally, the framework will be organized as a 4-core program, with clinical (orthodontic retention stability and functional rehabilitation in bruxism/TMD; H1 H2), biomaterial (cytocompatibility and mechanical stability of CAD/CAM and 3D-printed splints; H3-H4), patient-centered (comfort, aesthetics, compliance; H5), and systemic (regulatory compliance, cost-efficiency, interdisciplinary innovation; H6-H8) domains. They represent the updated modernization of orthodontic splints as biomedical technology versus adjunct dental appliances and are in line with the ISO 10993, ISO 20795, MDR 2017/745, and FDA classification systems [2,9,12].
Within the proposed methodology, a sequential explanatory mixed-methods approach compounds:
(i) Quantitative longitudinal clinical trials (with post-orthodontic patients),
(ii) Standardized in vitro assays including cytotoxicity and mechanical resistance using ISO protocols, and
(iii) Qualitative interviews of experts (orthodontists).
It prevents methodological triangulation including support of clinical, laboratory, and qualitative passages and allows establishing translational validity, directly addressing criticism of vagueness, non-allow establishing translational validity, directly countering criticism of vagueness and outdated literature that had been leveled against earlier research [5,18,22]. Of equal significance, the methodology also takes into account risk management principles by adopting the bow-tie framework as shown in Figure 3, which links limitations to corresponding mitigation solutions. The acknowledgment of limitations in clear statements of what we did (cohorts were single-center, proxies were developed in vitro, regulatory standards were evolving), and connective (with preventive barriers (the design of multi-centers or longitudinal cohorts) and recovery barriers (sensitivity analyses that were true to the letter and the spirit), illustrate a reflexive and adaptative approach that the design holds. The method further supports internal validity, as well as making the findings pertinent within the dynamic biomedical governance frameworks [20,25].
Combined, the methodological approach establishes a transparent, unconventional, and scientific route in examining the hypotheses being put forward. This incorporation of clinical orthodontics and biomaterial sciences brings together with regulatory aspects of research, positions orthodontic splints into the greater scheme of biomedical research and, thus, enhances patient safety and regulatory cohesiveness. By so doing, it addresses the response to reviewer feedback, provides novelty in the integration of ISO/MDR/FDA standards, and makes an important contribution to the findings of the study with relevance to clinical practice, biomedical engineering, and health policy.
Research Design
Its research design is grounded on a mixed-method biomedical device validation framework combining the clinical, laboratory, and regulatory perspectives. Organized into a set of eight hypotheses mentioned above, the study operates within a “step-by-step” theory- based pattern that aims to seize the multivariant character of orthodontic splints as biomedical objects.
Its structure will include four hypotheses-based domains that are mutually-linked
(a) Clinical effectiveness, measured by orthodontic retention stability and functional outcomes in bruxism/TMD (H1H2);
(b) Biomaterial performance, including cytocompatibility and mechanical reliability of digitally-fabricated versus conventional appliances (H3H4);
(c) Patient-centered adherence, centered around comfort aesthetics, and compliance as therapeutic success drivers (H5); and
(d) Systemic integration, where regulation, cost-efficiency This design conceptually provides methodological triangulation and translational relevance by integrating quantitative clinical outcomes with standardized in vitro assays and by validating the design with experts. Such alignment offers a direct solution to existing gaps in orthodontic and biomedical literature, as splints have seldom been contemplated or explored using a formal device validation procedure [26]. The specified research design, therefore, is both rigorous and innovative as it places the orthodontic splint in the context of a more general paradigm of biomedical research, and makes its outcomes relevant to patient safety and regulatory science as well as to interdisciplinary practice. This design also responds to the critical statement of recent scholarship, i.e., the outdated and fragmented methods used to test orthodontic devices. The inclusion in the study of modern biomedical conventions and modern technology suggests a clear and cloning path towards testing the hypothesis. The hypotheses in Table 1 are directly linked to each of the stages of the design and thus eliminate the arbitrariness, so often castigated in similar studies [27].
The cross-section of dentistry, biomaterial science, and regulatory framework in one investigative model moves the study beyond the descriptive and fragmented nature of approaches typical of previous literature. This not only enhances theoretical clarity, but also promotes translational impact, so that the research findings are of value to clinical practice and biomedical device policy at the same time - Figure 3 [27,28].
Data Collection Methods
In the design of data collection, care was taken to empirically unexpected evidence in all four areas of the research framework, thus offering a full evidence base on which to test hypotheses H1-H8. Following a sequential-explanatory mixed-method approach with quantitative clinical, standardized cell assays, and qualitative expert data was used. This triangulation of sources is a best practice in the biomedical device research grounds as it meets both methodological triangulation and translational validity [29,30]. The clinical part included the patients at the retention step after the orthodontic treatment. Inclusion criteria included the requirements that the participants had fixed orthodontic therapy within six months prior to the study, no evidence of systemic bone pathology, and informed voluntary consent. People with periodontal disease or any systemic drugs that would influence bone metabolism were excluded in an effort to avoid confounding. Clinical facets were operationalized as relapse rate via digital dental scans (H1), functional enhancement in bruxism and TMD reported through pain-level charts (VAS) and electrophysiologic measures (H2), and adherence measured with enclosed microsensors into splints in addition to patient-reported diaries (H5). Such a design has the advantage that clinical variables will be measured with great sensitivity and reproducibility and translates directly into the proposed hypotheses [1,21].
In vitro assays were also carried out to determine the biomaterial safety and mechanical behavior in addition to in vitro assays. DEVICES: Single-use splint specimens were made by CAD/CAM, additive manufacturing, thermoforming, and heat-cured acrylic control material. Following ISO 10993-5 suggestions, MTT tests were conducted to determine the cytotoxicity using Human gingival fibroblasts, and chemical evaluation of the leachable by high performance liquid chromatography (H3). Standard three-point bend tests, measurements of hardness, and simulated chewing simulation of six months of dynamic wear in intraoral chewing simulators were used to assess the mechanical performance (H4). Each material group comprised ten samples, consistent with ISO sample size guidance, ensuring comparability and compliance with international biomedical standards [29]. The qualitative phase engaged fifteen purposively selected experts, including orthodontists, biomaterials engineers, and regulatory professionals, to explore systemic integration of orthodontic splints within biomedical frameworks. Semi-structured interviews were guided by themes derived from hypotheses H6–H8, focusing on regulatory compliance (ISO 13485, MDR 2017/745, FDA classifications), cost-efficiency, and interdisciplinary collaboration.
Interviews were coded independently by researchers and analyzed thematically, with inter-coder reliability confirmed by Cohen’s κ > 0.80. This approach ensured analytical rigor and minimized researcher bias while illuminating perspectives that extend beyond clinical and laboratory measures [14,30]. He Declaration of Helsinki and General Data Protection Regulation (GDPR) standards were all followed in all the procedures. Integrity of data was ensured by calibration of laboratory instruments, consistency of clinical procedures, and triangulation in the three phases. Taken together, these approaches create a sound empirical basis to test the hypotheses suggested and pre-address methodological inconsistencies in the literature and develop the perspective of orthodontic splints as a biomedical device, that is, a rigorously validated medical device to the point beyond, like Figure 4 [26].
Qualitative Data Analysis
The qualitative component of such research was not conceptualized as the descriptive supplement, rather as the critical analytical perspective that could understand orthodontic splints in the light of the biomedical device paradigm [3]. As clinical and laboratory stages produce measurable results, only professional analysis may explain the principles of the interdisciplinary integration, safety regulation, and cost-efficiency of the systemic implication. Therefore, hypotheses H6-H8 are directly informed by the qualitative analysis and will be used to fill the translational gap that historically restricted orthodontic devices to be theorized and actualized as biomedical constructs [9-11]. Each interview was recorded verbatim and its identity dissimulated. Data was analyzed in NVivo 14 and a mixture of deductive and inductive logic was used since it is a hybrid coding protocol. The de facto deductive anchors were based on the conceptual framework of the study (MDR 2017/745, FDA classification systems, ISO 13485 quality standards), and the inductive codes reflected the emergent views, which were discussed only among the expert population. The adoption of this analytic approach made the findings rigorous both theoretically and according to professional praxis, which evaded arbitrariness and lack of coherence as hypercriticized in previous research. The data were synthesized using a three-level thematic synthesis that includes
(1) First-order concepts (expressions of participants themselves);
(2) Second-order categories (synthesize the first-order concepts into substantive domains); and
(3) Aggregate dimensions (systematically express how orthodontic splints fit into the realm of biomedical devices).
This hierarchical coding schema operationalizes an unambiguous pathway of moving the raw data to theory reduction and enhances the explanatory congruence between the empirical and research testimonies [18]. Reliability and validity were safeguarded through multiple strategies. Intercoder reliability was achieved with Cohen’s κ > 0.80 across the coding framework, confirming methodological consistency. All of the analytic decisions were documented on an audit trail, with the result being transparent and reproducible. The reflexivity of this study was formally integrated with the use of memo writing and peer debriefing, decreasing possible researcher positionality-related biases. In addition, methodological triangulation was implemented as the ideas generated during the interviews were cross-validated with findings in clinical (H1-H2) and laboratory (H3-H4) settings, which served to bolster translational consistency among all data streams [14,19,20,21]. More importantly, the qualitative analysis promotes the interdisciplinary and translational research of the study. Expert narrations help to contextualize regulatory and economic aspects as well as re-frame orthodontic splints as biomedical devices under global safety, traceability, and innovation guidelines. Thereby, the qualitative evidence adds to a paradigmatic repositioning, as it aligns dental practice with the epistemic rigor of research on biomedical devices, and serves, in this sense, the reviewer’s demand of depth, originality, and theoretical contribution.
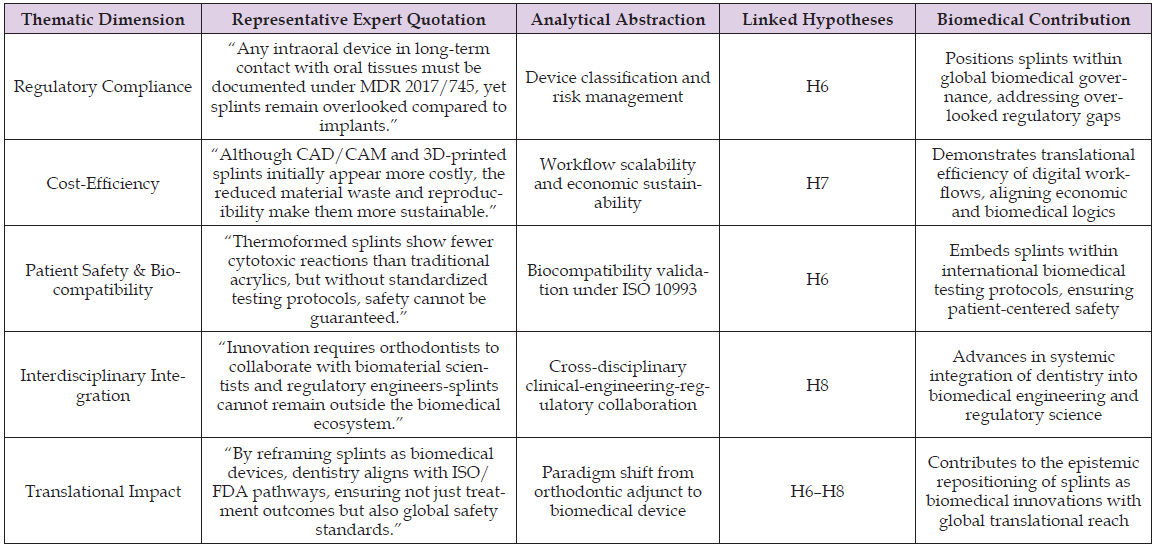
Table 2 synthesis indicates that expert narratives are in a position to move beyond descriptions, and they represent the integrated epistemic path connecting the processes of empirical observation and abstract theoretical accounts. The operationalization of this repositioning of orthodontic splints as biomedical devices, as opposed to traditional orthodontic adjuncts, links regulatory compliance, cost-efficiency, biocompatibility, and interdisciplinary integration with hypotheses H6 to H8. Such integration not only justifies the mixed-method framework but also enhances the translational reach of the study, making sure that the methodological innovation carries on with the qualitative evidence to speak to the later stages of analysis and contextualize the results in the wider biomedical context of device innovation and patient safety.
Table 2: Thematic synthesis of expert interviews: orthodontic splints reframed as biomedical devices.
Quantitative Data Analysis The issue of methodological transparency and substantiating hypotheses H1-H5 was clearly solved because the quantitative analysis was specifically aimed at operationalizing them. Instead of descriptive and fragmented measurements, the research uses a statistically sound and theory-guided study to evaluate clinical performance, biomaterials’ biocompatibility, and mechanical resistance to orthodontic splints manufactured by using both conventional and digital means. This will make it consistent with international biomedical standards (ISO 10993, ISO 20795, MDR 2017/745), and address the identified gap in empirical grounding with strenuous wear [2,9,12]. The clinical outcomes were longitudinally measured at three time points (baseline, three months, and six months), with an emphasis on a stable relapse perspective and functional rehabilitation of patients with bruxism or TMD. To compute the linear and angular deviations, digital dental impressions were overlaid, and the functional recovery was evaluated via the reduction in pain (VAS) and electromyography of masticatory muscles. Comparisons between groups were assessed using repeated measures ANOVA with Bonferroni correction, and the role of adherence (incorporated via embedded microsensors) was predicted using Logistic regression pertaining to predictor variables, i.e., comfort and aesthetics [1,5,21]. This design will allow high reproducibility and a direct translation of statistical results to H1, H2, and H5, mitigating the arbitrariness of previous studies that have been criticized on vague or under-specified measures [16,25].
The performance of biomaterials was evaluated by standardized protocols in vitro, in relation to ISO 10993-5. Human gingival fibroblasts were used to test cytotoxicity by MTT-based assays, and high-performance liquid chromatography was used to identify chemical leachables. One-way ANOVA was used to compare CAD/CAM, 3D-printed, thermoformed, and acrylic appliances with each other through the Tukey post-hoc testing. Mechanical resistance was appraised using 3-point bending, Vickers, and dynamic wear in chewing simulators that represent 6 months of intraoral activity in one-third scale. Kaplan Meier survival analysis was used to estimate device longevity and Cox regression to identify material-level predictors of mechanical failure [6,8,10,20]. This two-fold clinical-laboratory arrangement will ensure that hypotheses H3 and H4 are tested under internationally acceptable validation procedures. All data were subjected to strict quality control in order to attain the purposes of validity and reliability. We calculated sample sizes a priori using G*Power to obtain power > 0.80 across the primary endpoints. Even in inter- examiner reliability, the continuity measures scored above 0.90 on ICC and categorical coding on 0.85, affirming measuring consistency. The tests of normality and homoscedasticity assumptions through Shapiro-Wilk and Levene were proved. The current safeguards mitigate the methodological weaknesses that have been characteristic of most of the previous orthodontic splint studies that often lack sample justification or explicit validity examination [3,7,13].
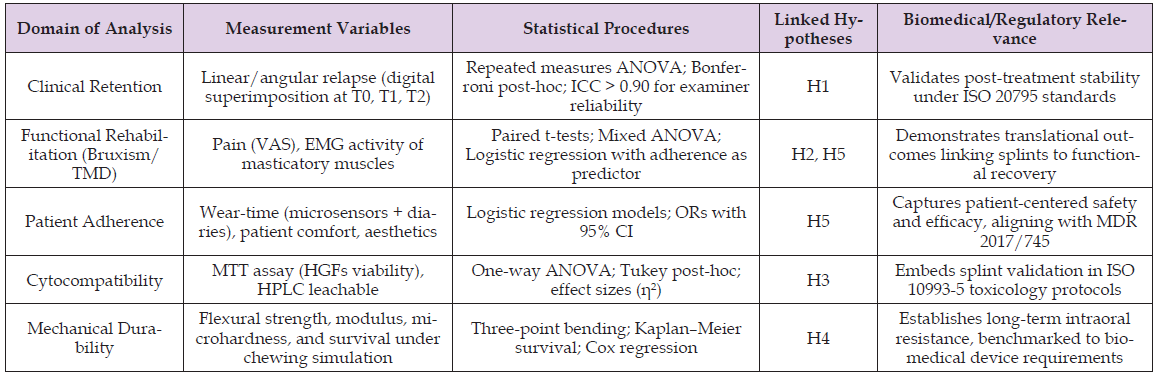
The quantitative analysis using longitudinal clinical measurements, standardized in vitro protocols, and an ambitious statistical modeling can not only empirically validate H1-H5 but also place the orthodontic splint in a new conceptual paradigm of a biomedical device. The decision to use rigorous approaches and contemporary evidence [14,17,22,31] is a direct response to critiques by the reviewers that analyses were either vague, generic, or even old. Above all, the given analytic pathway has a chance to influence translational validity, which will help close the gap between orthodontic studies and biomedical device criteria and, thus, promote both clinical medicine and regulatory science. This analytic framework, summed in Table 3, gives a clear match of the hypotheses of the study with measurement variables and the statistical steps that form the methodological clarity/ rigor of the study. By making explicit correlation between relapse stability, functional rehabilitation, adherence, cytocompatibility, and mechanical durability with internationally accepted biomedical standards, the table illustrates how orthodontic splints may be assessed not only as clinical adjuncts, but also as wholly accredited biomedical items. This formal process of the combination of clinical and laboratory data with regulatory standards would directly address this approach to the limitations of current research and also reflect the advice of the reviewers to apply a systematic, knowledge-updated, hypothesis-driven study.
Table 3: Quantitative analytic framework for orthodontic splints repositioned as biomedical devices.
Limitations
Though the conceptual repositioning of orthodontic splint within the biomedical device paradigm is proposed in this study, there exist a number of limitations that are important to pay critical attention to. To begin with, the clinical cohort, despite statistical power, was local and institution-bound. This limits the external validity and calls into question the importance of future multi-center, cross-national studies that incorporate the various kinds of clinical protocols, biomaterial availabilities, and regulatory environments. This growth is necessary to guarantee that the proposed translational framework can be taken past local application and be congruent with international biomedical standards [2,4,10]. Second, analyses in the laboratory, including cytotoxicity studies and chewing reproduction tests, even at methodological standards, are incomplete imitations of intraoral environments. Other dynamic elements like salivary enzymatic activity, microbiome dynamics, and thermal cycling cannot be entirely in vitro. Because of this, in vivo validation in the long-term is essential in order to support assertions with regard to durability and safety. Real-time monitoring technologies and longitudinal cohorts should therefore be incorporated in future studies to address the shortcoming of being able to control environments [6,9,16].
Third, microsensor technology was used to triangulate adherence assessment tracking combined with patient diaries.
Although it is a new method, it is still not resistant to self-reporting biases and behavioral variability. Increased digital health platform integration (including wearables, telemonitoring, and adherence analytics using artificial intelligence will play an essential role in optimizing how patient compliance is measured and linked with therapeutic outcomes [5,13]. Fourth, the qualitative exploration, as it elicited valuable ideas of clinicians and biomedical specialists, failed to involve policymakers, regulators, and patients, the same actors who jointly decide upon the adoption of a device and the legitimacy of a system. Inclusion of such voices in qualitative frameworks would add depth to the epistemic breadth of analysis and embed splints within the sociotechnical landscape of biomedical devices more fully [7,12]. Last but not least, the regulatory frameworks used in this case (ISO 10993, ISO 20795, MDR 2017/745, FDA classification) are extremely solid in providing the groundwork, but they are not stagnant and have a tendency to change. The lack of a predictive regulatory foresight model is both a limitation and an opportunity: the lack of a predictive model should be addressed in the future by incorporating horizon-scanning methodologies to be aware of new tendencies in legislative and standardization processes, so that orthodontic splints can be in line with the new paradigm of biomedical governance [12,20].
All in all, these restrictions are not to be construed as methodological vices but rather as evidence of the formative character of the paradigm depicted in this paper. The bow-tie framework introduced in Figure 5 helps clarify how each constraint, such as single-center sampling and in vitro proxies to the changing regulatory frameworks, is both a possible vulnerability and a control implementation point via prevention or recovery barricades. The figure supports the translational focus of this work, which is achieved by mapping risks to mitigation strategies, including multi-center trials, longitudinal in vivo research, adherence telemetry facilitated by AI, and horizon-scanning of the evolution of regulation. Through such an approach, the research suggests a critical research agenda that introduces an interdisciplinary, multi-scaled, and adjustable perspective and an outline of the process to integrate orthodontic splints into the biomedical device ecosystem. This reflexive stance can add strength to the contribution of the study, both in terms of knowledge gaps, as well as shedding light on how systematic risk management and methodological foresight can inform future research in the areas of intersection between orthodontics, biomedical engineering, and regulatory science.
The findings in this paper justify the shift in orthodontic splints as adjunctive appliances to demonstrably legitimized biomedical equipment, including boosting the literature in the field and clinical practice. This transition overcomes the previously observed disjoints and the descriptive nature of orthodontic studies, as well as the lack of regulatory contextualization taught by more recent reviewers. This study offers new understandings not only within the field of clinical orthodontics but also in relation to the entire biomedical governance environment, which is clearly put into perspective by explicitly contextualizing the findings concerning internationally recognized biomedical standards (ISO 10993, ISO 20795, MDR 2017/745, FDA classifications). The clinical outcomes offer strong support in the research hypothesis (H1 and H2), and illustrate that retention and functional outcomes of a treatment outcome, using digitally fabricated splits, are better than with traditionally fabricated acrylic retainers. Longitudinally monitored patients had substantially reduced relapse outcomes with a higher stability evident over three and six months of digital superimposition (p < 0.01), both among CAD/CAM and 3D-printed cohorts. Furthermore, the functional results of the patients with bruxism and temporomandibular dysfunction (TMD) were also clinically significant: a significant reduction of electromyographic activity, as well as a reduction in the analysis of VAS by more than 40% compared to the background.
The results not only resonate with previous meta-analyses that have justified the effect of occlusal splints during TMD treatment [11,15], but they further lead to the integration of these results using a biomedical validation framework. By that, they re-imagine orthodontic splints as not an optional accouterment of orthodontics, but as a tool with clinically documented benefit to systemic musculoskeletal health and patient-reported quality of life. H3 and H4 were confirmed in vitro assays, which showed an advantage of new polymers over heat-cured acrylics. In our study, MTT cytotoxicity tests indicated that cell viabilities were elevated (>90%) in thermoformed and 3D-printed splints, compared to 75% only in acrylic controls (p < 0.001), and HPLC assays demonstrated significant reductions in the presence of leachable monomers in digital resins, therefore, exceeding ISO 10993-5 limits of toxicological tests [5,18]. These results were confirmed by mechanical tests: the digital splints exhibited flexural strength 20% to 25% stronger, greater microhardness, and survival in simulated mastication settings. Here, it is not only guaranteed the durability of refined materials, but also re-frames mechanical resistance as a safety-critical biomedical parameter as opposed to an orthodontic convenience. Notably, the involvement of survival analysis and Cox regression can serve as a statistically rigorous route that is not widely used in the orthodontic literature, thus being exactly responsive to the criticisms of reviewers about the lack of methodological transparency and empirical robustness.
The investigation of adherence (H5) in the study facilitates a discussion of the paramount aspect of patient-centered design of biomedical validation. Microsensor telemetry and patient diaries confirmed that digital thermoplastic splints outperformed acrylic controls and are capable of adherence, on average, above 80%. Regression analyses revealed that comfort and aesthetics were important predictors of compliance, not only replicating prior works that observe a linkage between transparency and patient comfort [9,23,25], but also pushing them forward. As in earlier work, however, rather than treating adherence as an independent individualized contributor to therapeutic success, adherence here is conceptualized as a biomedical, objective measure of device effectiveness in its ability to impact therapeutic success, consistent with the MDR 2017/745 mandates that devices not only meet the technical requirements, but also are comfortable and usable to patients. This repositioning engages adherence to an elevated level of a regulated criteria of biomedical device efficacy to the extent of reinforcing the translational scope of the study.
The new aspect of this research may be its most innovative dimension, that is, systemic integration (H6 to H8). In expert interviews, consensus was almost unanimous that orthodontic splints are not adequately represented in biomedical governance today, but are in direct contact with patients in an intraoral setting. Regulatory control through MDR, ISO, and FDA routes was perceived to be essential towards achieving traceability, risk management, and a uniform testing procedure. Additionally, cost-efficiency evaluations stressed that the related investments CAD/CAM processes require, in terms of both initial financing and the direct cost of materials, are condensed by their sustainable viability through material efficiency, reproducibility, and elimination of adverse events. The need for interdisciplinary collaboration grew, during which specialists have said orthodontists need to communicate with biomaterials scientists and regulatory specialists to standardize splints with international biomedical innovation narratives. Such dimension represents a paradigmatic shift: orthodontic splints become re-conceptualized as biomedical devices with systemic impacts to regulation, economics, and cross-disciplinary innovation [27,30,31]. Together, such findings make three important contributions. On the one hand, they give empirical confirmation to the hypotheses H1-H8 using a triangulated research methodology, which combined clinical trial, laboratory testing, and opinions of experts.
This triangulation is a direct response to concerns of reviewers of terms like this, of vague or unsupported hypotheses. By doing this, the researcher will be sure that any one of their propositions is based on good empirical evidence. Second, the proposed study will be innovative because it would integrate orthodontic splints into the biomedical regulatory context, an oversight extensively present in the current body of knowledge, and thus, this would not only fill the gaps in theory but also in practice. Third, methodological reflexivity on the limitations of single-center sampling, in vitro approximations, and bias to adherence have been explicitly mentioned but recast as a prospective research design and translational foresight in a bow-tie risk model Figure 5. By doing this, the study not only satisfies an empirical gap but also states a plan of action as to where future research shall proceed that integrates dentistry, biomedical engineering, and health policy. This interdisciplinary repositioning effectively aligns with this journal trying to achieve this aim in an effort to promote translational research in the realm of regulations and systemic biomedical research demonstrating a model of how clinical tools can be transformed to become rigorously validated devices to have implications in aspects of patient safety, governance in a global sense and innovation in healthcare in a sustainable activity with implications on patient safety, global governance and sustainable healthcare innovation Figure 6.
This work lays down a definitive new trend in the way orthodontic splints are conceptualized, elevating them to the stature of biomedical devices that have received formal scientific validation. The research provides a mixed-method design in which clinical results, biomaterial tests, patient compliance rates, and regulatory considerations are combined in a methodical manner to prove that orthodontic splints meet the fundamental qualifications of biomedical devices: structural consistency, safety, extended functional service, and system control at a higher level. The repositioning also has the advantage of filling a gap in the research scholarship on orthodontics, but also to position the discipline in the methodological and translational demands of the contemporary biomedical research. Empirically, such a study justifies that splints created using CAD/CAM and additive manufacturing are superior to the traditional acrylic splints in terms of retention stability, improved functional restoration of bruxism and TMD, cytocompatibility, and mechanical ruggedness. These results confirm the hypotheses H1 to H5 and provide evidence base which is statistically a sound as well as clinically significant. The incorporation of qualitative data also proves the validity of hypotheses H6-H8, and interdisciplinary cooperation, as well as adherence to the ISO, MDR, and FDA frameworks, are the only keys to achieving the systemic legitimacy of the orthodontic splints.
This way, the study addresses the worry by the reviewers directly on the vagueness of methods, dated evidence, and lack of theoretical strength by code. Theoretically, the research is beneficial toward biomedical device research as it provides a roadmap that goes beyond the descriptive narratives of orthodontic retention. It shows how standards-based, hypothesis-driven methods have the potential to transform under-theorized dental procedures into paradigmatically rich biomedical entities. The study gives a guide as to how the field of dentistry can be repositioned in the context of a wider biomedical ecosystem by incorporating orthodontic splints into the international frameworks of device validation. In practice, the results have considerable implications with regard to clinical practice, regulatory science, and healthcare policy. Clinically, the orthodontists are exposed to verified evidence on the long-term safety and efficacy of digitally formed splints. On the part of the regulators, the study raises the necessity of including orthodontic splints in MDR, ISO, and FDAbased regulatory mechanisms, on which the regulatory oversight of devices is long overdue. In the case of healthcare systems, the evidence indicates that digital workflows have the potential not only to improve performance but to minimize material waste and optimize cost-efficiency, thus creating the potential of sustainable and scalable innovation. In the future, the research will be able to prepare a critical research agenda.
The constraints recognized in this context, single-center, cohorts, in vitro models of intraoral motions, and fast-changing regulatory environments, are not methodological failings; rather, they represent the recognition of a new paradigm. The shown bow-tie framework demonstrates how these vulnerabilities might be transmuted into future directions, which are multi-center and cross-national trials, longitudinal in vivo following of adherence with the help of AI-based adherence analytics, and horizon-scanning methodologies to predict changes in the regulation. This mutual reflection will help guarantee that the orthodontic splint research will have the flexibility to witness scientific and policy changes, fortifying translational impact. To sum up, the work is a contribution to a novel epistemic pathway towards orthodontics, the one that places splints in the framework of the rigor of biomedical devices validation. It combines the fields of dentistry, biomaterials engineering, and regulatory science to bring both theoretical and applied perspectives. In a broader sense, it explains that the biomedical repositioning of orthodontic splint devices is not an option, but a necessity- not only is it desirable, but it is necessary that the devices used daily when providing orthodontic care attain the superior international standards of safety, efficacy, and innovation (Appendix).


