Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
N Mihnev, M Cholakova* and I Staikov
Received: August 25, 2025; Published: September 02, 2025
*Corresponding author: M Cholakova, Acibadem City Clinic UMHAT Tokuda Hospital, Bulgaria
DOI: 10.26717/BJSTR.2025.63.009843
Misdiagnosis of psychogenic seizures as epileptic seizures is very costly. It is known that patients with psychogenic pseudoepileptic seizures very often seek medical help, which, in addition to unnecessary prescription of anticonvulsants, further increases the cost of treatment. The indirect costs associated with loss of work capacity are significantly higher. Six months after diagnosis with video-EEG monitoring, hospitalization costs were significantly reduced.
Keywords: Epilepsy; Video-EEG; Pschogenic Seizures
About 30% of patients with drug resistant epilepsy are misdiagnosed. The most common reasons are: the patient may have psychogenic attacks, use of the wrong medication or patients have low compliance to treatment. This necessitates the introduction of the concept of pseudo-resistance. In pseudoresistance, non-response is due to diagnostic errors [1]. Misdiagnosis of psychogenic seizures as epileptic seizures is very costly. In the United States, the annual direct costs are between $650,000,000 and $4,000,000,000 [2]. It is known that patients with psychogenic pseudoepileptic seizures very often seek medical help [3], which, in addition to unnecessary prescription of anticonvulsants, further increases the cost of treatment. The indirect costs associated with loss of work capacity are significantly higher. One large study showed that only 20% of patients were still working at the time of diagnosis [4]. 6 months after diagnosis with video-EEG monitoring, hospitalization costs were significantly reduced [5]. Routine EEG studies are often normal between seizures. According to different authors, between 30 and 70% of patients, depending on the type of epilepsy, have abnormal electroencephalograms during routine recordings [4,5]. The diagnostic value of the method is increased by using techniques that stimulate the appearance of electrical responses [6]. Recording of ictal or interictal EEG changes in some patients suspected of epilepsy is impossible, even with repeated routine recordings.
Ambulatory EEG monitoring for an extended period increases the likelihood of obtaining an abnormal EEG or recording during an attack. In addition, it provides reliable data for the evaluation of patients with suspected syncope, transient ischemic attacks, psychogenic seizures, and unclear epileptic seizures [7]. Short-term video-EEG monitoring can be performed in an outpatient setting and lasts 1-12 hours. This study is appropriate for patients with frequent and provoked seizures [8]. The disadvantages of this method are the limited duration of monitoring, which does not allow the recording of epileptiform activity, and the need for staff to monitor the patient in the laboratory. Reducing antiepileptic drugs before the study can be dangerous and ineffective and is not appropriate for short-term video-EEG monitoring.
Video-EEG recording with duration 1-2 hours, routine EEG (EEG-R), activation procedures- photostimulation, hyperventilation, sleep, sleep deprivation, saline ingection.
Comparison of Psychogenic Non-Epileptic Seizures (PNES) and Epileptic Seizures
A total of 97 patients were studied, of whom 50 were women, 47 were men with a mean age of 36.9 years (SD±14.268). The mean age of patients with epileptic seizures was 38.12 years (SD = 14.962), whereas patients with PNES had a mean age of 43.67 years (SD = 13.351). Although PNES patients tended to be older than those with epileptic seizures, Levene’s test did not indicate statistical significance (Table 1).
Gender Distribution
Among patients with epileptic seizures, 6 (35.3%) were female and 11 (64.7%) were male. In contrast, the majority of PNES patients were female (7 patients, 87.5%), with only 1 male (12.5%). These findings demonstrate a marked female predominance among PNES patients. In contrast, no significant gender differences were observed among epilepsy patients. Fisher’s exact test confirmed statistical significance (p = 0.047) (Table 2).
Duration of Seizure History
Patients were categorized into four groups according to seizure duration:
1. 2-5 years
2. 6-10 years
3. 11-20 years
4. 21 years
PNES patients typically had a shorter seizure history, with no cases exceeding 10 years. Conversely, most epilepsy patients monitored with video-EEG had a seizure history of more than 11 years. Fisher’s exact test indicated statistical significance (p = 0.015) (Table 3).
Table 3: Distribution of seizure duration in patients with psychogenic and epileptic seizures recorded during video-EEG monitoring.

Seizure Frequency
Patients were divided into four groups based on seizure frequency during the preceding 3 months:
1. 2-5 seizures
2. 6-10 seizures
3. 11-20 seizures
4. 21 seizures
The probability of capturing a seizure during video-EEG monitoring increased with seizure frequency in both groups, although the difference was not statistically significant (p = 0.910). When analyzed at the population level, however, the results differed: the majority of epilepsy patients reported 2-5 seizures in the last 3 months (50.5%), followed by 6-10 seizures (18.6%), >21 seizures (18.5%), and 11- 20 seizures (12.4%). In contrast, 87.5% of PNES patients and 30.9% of epilepsy patients experienced more than 11 seizures in the last 3 months, a statistically significant difference (p = 0.021) (Table 4).
Table 4: Seizure frequency over the last 3 months in patients with psychogenic and epileptic seizures recorded during video-EEG monitoring.

Clinical, Imaging, and EEG Characteristics
Patients were compared according to seizure semiology, medical history, neuroimaging, and EEG findings (Table 5).
Table 5: Comparison of PNES and epileptic seizures according to medical history, imaging, EEG, and semiological characteristics.
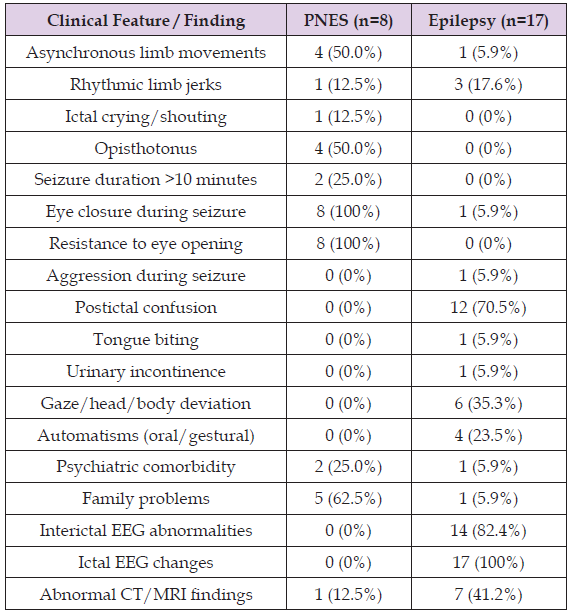
The graphs indicate that asynchronous limb movements during seizures were observed in 50% of PNES patients but only in 5.9% of those with epilepsy. Rhythmic limb jerks, ictal crying, and postictal confusion were documented in 12.5% of PNES patients and in 5.9% of epilepsy patients. Opisthotonus was reported in 50% of PNES cases and in none of the epilepsy cases. Seizures lasting longer than 10 minutes were recorded in 25% of PNES patients, whereas no epilepsy patient monitored with video-EEG demonstrated such prolonged events. All PNES patients exhibited eye closure during seizures, consistently resisting attempts at passive eyelid opening, in contrast to epilepsy patients, of whom only 5.9% showed eye closure without resistance. Postictal confusion was present in 12.5% of PNES patients but in 70.5% of epilepsy patients. The relatively low percentage of postictal confusion in the epilepsy group may be explained by the inclusion of patients with myoclonic seizures, absence seizures, and frontal lobe seizures, in which postictal confusion is typically absent or minimal. Tongue biting (5.9%), urinary incontinence (5.9%), gaze or head/body deviation (35.3%), and oral or gestural automatisms (23.5%) were reported exclusively in epilepsy patients. Interictal abnormalities (82.4%) and ictal EEG changes (100%) were also found only in epilepsy patients. Abnormal CT/MRI findings were more frequent among epilepsy patients (41.2%) compared with PNES patients (12.5%).
Taken together, these findings highlight several clinical and paraclinical features that may assist in differentiating PNES from epileptic seizures in routine practice. Features strongly suggestive of PNES include asynchronous limb movements, prolonged seizure duration (>10 minutes), eye closure with resistance to opening, and the absence of postictal confusion. Conversely, tongue biting, incontinence, gaze deviation, automatisms, and the presence of EEG abnormalities or structural brain lesions are more characteristic of epileptic seizures. Importantly, while no single clinical sign is entirely pathognomonic, the combination of semiological features, EEG data, and neuroimaging significantly improves diagnostic accuracy. These observations are consistent with previous reports. For example, eye closure with resistance to passive opening has been repeatedly identified as one of the most reliable clinical indicators of PNES [8,9], whereas postictal confusion, tongue biting, and incontinence are more typical of epileptic seizures [10,11]. Earlier studies have also emphasized that PNES events often last longer than epileptic seizures [12], which aligns with the prolonged duration observed in our PNES group. Conversely, ictal and interictal EEG abnormalities remain the gold standard for confirming epilepsy [13-16], and their absence in PNES patients further supports the functional, rather than structural, nature of these events.
Our results therefore reinforce established diagnostic criteria and provide additional evidence for the clinical value of combining semiological observation with neurophysiological and imaging studies in distinguishing PNES from epilepsy.


