Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jiazhen Ren1, Yujin Gu1, Yanglin Guo1, Ruben K. Dagda2, Yuri N. Utkin3, Edward S. Gasanoff1,4*
Received: July 21, 2025; Published: August 13, 2025
*Corresponding author: Edward S Gasanoff, Advanced STEM Research Center, Beijing Chaoyang KaiWen Academy, Beijing 100018, China, Belozersky Institute of Physico-Chemical Biology, M.V. Lomonosov Moscow State University, Moscow 119991, Russia
DOI: 10.26717/BJSTR.2025.62.009810
This study characterizes the substrate specificity and membrane interactions of heterodimeric phospholipase A₂ (HDP-2) from Vipera nikolskii venom through combined biochemical and computational approaches. Using model membrane systems comprising pure phospholipids (phosphatidylcholine, cardiolipin, phosphatidylserine, and sphingomyelin) and physiologically relevant mixed membranes, we demonstrate that HDP-2 exhibits distinct hydrolytic preferences. The enzyme shows greater activity toward pure cardiolipin than phosphatidylserine, while in mixed membranes this preference reverses, with enhanced hydrolysis observed in phosphatidylcholine- phosphatidylserine systems compared to phosphatidylcholine-cardiolipin membranes. Notably, sphingomyelin-rich membranes resist hydrolysis unless combined with phosphatidylcholine. AutoDock simulations reveal the structural basis for these observations, showing productive binding of phosphatidylserine, phosphatidylcholine, and cardiolipin to HDP-2’s active site, with optimal positioning of their sn-2 ester bonds for catalysis. In contrast, sphingomyelin binds peripherally, explaining its resistance to hydrolysis. These findings align with the enzyme’s physiological targets, suggesting preferential attack on cardiolipin-rich mitochondria of cardiomyocytes and phosphatidylserine-exposing membranes in autophagy, while sparing sphingomyelin-rich neural membranes.
Compared to its homolog HDP-1 (known to affect cardiac contractility), HDP-2 appears optimized for disrupting cardiomyocyte membranes during envenomation. The differential hydrolysis of phosphatidylserine versus sphingomyelin suggests dual pharmacological roles: phosphatidylserine may amplify apoptotic signaling through enhanced phospholipase A₂ activity, while sphingomyelin’s resistance to hydrolysis may provide inherent neuroprotection against excessive membrane degradation. These results highlight how membrane lipid composition and packing density critically regulate HDP-2’s neurotoxic potential, with important implications for understanding viper envenomation pathophysiology and developing targeted therapies.
Abbreviations: PLA₂: Phospholipase A₂; sPLA₂s: Secretory PLA₂s; CL: Cardiolipin; PS: Phosphatidylserine; SM: Sphingomyelin; PC: Phosphatidylcholine; LUL: Large Unilamellar Liposomes; SD: Standard Deviation; CL: Cardiolipin; HDP-2: Heterodimeric Phospholipase A₂
Phospholipase A₂ (PLA₂) enzymes, which hydrolyze the acyl chain at the sn-2 position of phospholipids, are key components of snake venoms [1]. These enzymes initiate diverse pathophysiological processes with significant pharmacological implications [1]. Among these, secretory PLA₂s (sPLA₂s) of group IIA are notably implicated in inflammatory responses associated with disease states [2,3]. Heterodimeric PLA₂s within this group have attracted particular attention due to their neurotoxic effects [4,5]. Beyond neurotoxicity, these enzymes target immunocompetent and blood cells. For example, crotoxin from Crotalus durissus terrificus venom suppresses spleen cell proliferation and cytokine secretion [6], viperotoxin from Vipera russelli formosensis exhibits minimal anticoagulant activity [7], and heterodimers from Vipera nikolskii venom induce blood clotting while reducing platelet aggregation [8]. Additionally, Vipera nikolskii heterodimers aggregate acidic phosphatidylglycerol membranes but not neutral phosphatidylcholine membranes [9]. Heterodimeric PLA₂s occur in only a few snake venoms [1]. Alongside Vipera nikolskii, vipoxin is found in Vipera ammodytes [10] and crotoxin in Crotalus durissus terrificus [11]. Vipera nikolskii venom is unique in containing two heterodimeric PLA₂s, HDP-1 and HDP-2, structurally analogous to other snake venom PLA₂s [8]. While the amino acid sequences of their subunits are homologous, only one subunit (HDP-1P or HDP-2P) is enzymatically active, whereas the other (HDP-1I, identical in both enzymes) is inactive [9,12,13].
The catalytic subunits are basic, while the non-catalytic subunit is acidic, with both non-covalently bound [9,12]. The molecular mechanism of heterodimeric PLA₂ toxicity remains poorly understood. Membrane binding by the active subunit triggers heterodimer dissociation [7,14], enhancing PLA₂ activity. Notably, toxicity is not solely dependent on hydrolysis; certain viper venom heterodimeric PLA₂s possess a distinct surface site (outside the catalytic center) critical for toxic effects [6,15,16], though its structure remains uncharacterized. HDP-1 and HDP-2 share homology with neurotoxic group II sPLA₂s [12]. HDP-1 can alters rat heart papillary muscle contractility [17], though its mechanism is unclear, while HDP-2’s effects remain unstudied. PLA₂ may influence cardiac contractility via two pathways:
a) Hydrolysis of cardiomyocyte membrane phospholipids or via b) Hydrolysis of cardiac neuron membranes.
Cobra venom cardiotoxin (which similarly affects heart muscle contractility) targets mitochondrial cardiolipin in cardiomyocytes [18]. A recent study employed HDP-2 in model membranes to simulate aberrant PLA₂ activity potentially linked to neurodegeneration [13]. This study investigates whether HDP-2 affects heart muscle contractility via hydrolysis of cardiomyocyte-like (CL-rich) or neuron- like (SM-rich) model membranes. Cardiomyocytes are rich in mitochondria. In cardiomyocytes, the number of mitochondria is several times higher than in any other type of cell. Cardiolipin (CL) is a phospholipid which is exclusively found in mitochondrial membranes [19]. Given that CL is the most abundant phospholipid in membranes of cardiomyocytes, so we used CL in this study as a representative molecule of membranes of cardiomyocytes to be potentially targeted by HDP-2 in this study. Aslo we employed SM in this study to model targeting nerve membranes by HDP-2 as SM is a characteristic phospholipid in membranes of nerve cells is sphingomyelin (SM) [20-22]. In this study we also used phosphatidylserine (PS), as PS is a phospholipid which flips to the outer layer of plasma membranes in states of pathology and is found in all types of eukaryotic cells [23], including cardiomyocytes and nerve cells. We have also used in this study phosphatidylcholine (PC) - the most abundant phospholipid found in plasma membranes and membranes of organelles in any type of cells [24]. The model membranes we used in this study were made of either pure CL, SM, PS or PC. We have also used membranes made of PC enriched with 20 mol% of either CL, SM or PS. For measuring hydrolytic activity of HDP-2 in samples of model membranes we utilized FUJIFILM Wako’s NEFA kit. To predict whether CL, SM, PS or PC can form a tight complementary docking structure with the active center of HDP-2, we performed in silico simulations of interactions between CL, SM, PS and PC with the digital coordinates of HDP-2 using AutoDock program.
The overall aim of this study was to determine whether HDP-2 can act as neurotoxin by preferentially hydrolyzing SM in model membranes (suggesting potential ability to affect contractility of nerve cells of heart muscles) or whether HDP-2 can act as cardiotoxin by preferentially hydrolyzing CL in model membranes (suggesting that HDP-2 can affect contractility of cardiomyocytes). In addition, this study aimed to examine whether PS can enhance hydrolytic activity of HDP-2, which should shed more light on potential physiological role of PS in triggering autophagy in states of disease.
Materials and Equipment
HDP-2 enzyme, with a purity of 98.5%, was isolated from Vipera nikolskii viper venom following the protocol described previously [12]. Model bilayer liposomal membranes were prepared using lipids of ≥99.5% purity from Sigma Chemical Co. (St. Louis, MO, USA): egg yolk L-α-phosphatidylcholine (PC), porcine brain sphingomyelin (SM), Escherichia coli cardiolipin (CL) and bovine brain L-α-phosphatidyl- L-serine (PS). Additional reagents included chloroform, methanol, EDTA, CaCl₂ and 0.1 M Tris-HCl pH 7.4 buffer (Thermo Fisher Scientific Inc., Waltham, MA, USA), deionized-distilled water (dd-H₂O) (XiZhiMeng Co., Ltd., Guangdong, China). FUJIFILM Wako’s NEFA kit (Lexington, MA, USA) was used for measuring the HDP-2’s hydrolytic activity.
The equipment used in this study included: Jinghua Instruments UV-Visible spectrophotometer JingHua 752 (Shanghai, China), Yetuo Technology ultrasonic disperser Yt-JY96-IIN (Shanghai, China), Microcentrifuge TGL-16B (Shanghai 3 Anting Co., Shanghai, China), Vacuum pump TW-1M (Tingwei Co. Ltd., Wenling, China), Rotary evaporator RE-52AA (Shanghai Yarong Biochemical Instrument Co., Ltd., China), High-precision balance FA2004 (Shanghai Maiyi Ltd. Co., China), Thermostat SHHW.21-420 (YongGuangMing Medical Instrument Ltd. Co., Beijing, China), Hamilton 100-microliter (μL) syringe (Hamilton Co., Boston, USA) and PC HYLR-WFQ9 with installed AutoDock Vina (Version 4.2) and MGL Tools Python Molecular Viewer (Scripps Research Institute) programs.
Methods
Stock solution of HDP-2 (Mr 13.827 kDa) was prepared in 0.10 M Tris-HCl buffer (pH 7.4) with enzyme concentration of 0.54 M. Stock solution of EDTA was 1.0 M EDTA in double-distilled water (dd-H2O). Stock solutions of either CL, SM, PS or PC in methanol/chloroform (50/50% by volume) had concentrations 1.0 M. Samples of liposomes that model cardiomyocyte membranes were comprised of either pure CL or PC+CL at 4:1 molar ratio at 5 mM overall phospholipid concentration. Samples of liposomes that model nerve cell membranes were made of either pure SM or PC+SM at 4:1 molar ratio at 5 mM overall phospholipid concentration. Samples of liposomes that model membranes of any cell type were made of either pure PS or PC+PS at 4:1 molar ratio at 5 mM overall phospholipid concentration. To prepare large unilamellar liposomes (LUL) made of either pure CL, SM, PS or PC, or PC enriched with 20 mol% CL, SM or PS, the ether evaporation method [25] was used with minor modifications. Briefly, 50 μmol of phospholipids from methanol/ethanol stock solutions were dried in round-bottom flask with Vacuum pump TW-1M at 10 Pa for 25 minutes at room temperature dissolved in 10 mL of diethyl ether. The mixture was gently swirled to ensure complete lipid dissolution. 10 mL 0.10 M Tris-HCl buffer (pH 7.4) was added to the lipid-diether solution. The two-phase system was sonicated on ice bath for 5 min with ultrasonic disperser Yt-JY96-IIN at 18 kHz frequency to form a homogeneous water-in-ether emulsion.
The flask was then attached to a rotary evaporator RE-52AA, and the organic solvent was gradually removed under reduced pressure while maintaining the flask in a temperature-controlled water bath (35–37°C) for 60 min. As the ether evaporated, the lipid molecules formed a thin film along the inner surface of the flask, followed by hydration and spontaneous formation of LUVs. The resulting liposome suspension was further homogenized by extrusion through a polycarbonate membrane (e.g., 0.2–1.0 μm pore size) to yield LULs with diameters ranging from 100 nm to 1 μm. 1 mL of each LUL sample was separately incubated for 20 min at 37°C in thermostat with HDP-2 at HDP-2/phospholipid molar ratios 0.001, 0.002, 0.003 and 0.004. Control LUL samples were incubated without HDP-2. Prior to incubation, 10 μL of FUJIFILM Wako’s NEFA kit colorless solution with coenzyme-A was added to each liposome sample. During phospholipid hydrolysis by HDP-2, a colorless solution turns purple due to coenzyme- A acylation by free fatty acids. After incubation, phospholipid hydrolysis was stopped by adding 100 μL EDTA stock solution and vortexing for 2 min. Phospholipid hydrolysis by HDP-2 was quantified by measuring optical density of liposome samples spectrophotometrically at 550 nm in arbitrary units. Each reported data point is the average optical density value derived from the three independent trials, with a standard deviation of no more than ±5% of the average.
The binding of phospholipids on the molecular surface of HDP-2 was performed to analyse whether phospholipids can dock with the active site of HDP-2 was investigated by using AutoDock Vina (Version 4.2) [26]. In brief, the digital coordinates for atoms of HDP-2 were taken from the protein data base using PDB code 2I0U. The PITP-PC complex (PDB code 1T27) provided the digital coordinates of PC’s atoms, while digital coordinates for CL, SM, and PS were generated using Chem3D and ChemDraw. The GROMOS force field was applied for energy minimization and charge validation of the docked molecules. All molecules were hydrated at pH 7.4 before docking. The entire surface of HDP-2 was included for docking simulations, with the following grid box parameters for the center: x = 25.647, y = 9.486, 41 = 10.512 and the size: x = 39.801, y = 53.401, z = 42.552. Phospholipids were assigned rotatable bonds to allow for flexible docking. HDP-2 was docked as a rigid molecule. Python Molecular Viewer (MGL Tools, The Scripps Research Institute) was used to visually identify and analyze hydrogen, ion-polar and ionic bonds in docked conformations. Each docking experiment was performed in triplicate, and no variation in the binding sites and the mode of intermolecular bonding was observed on the molecular surface of HDP-2 across the three runs for each docking set.
Statistical Analysis
Each hydrolytic activity data point was obtained from experiments conducted in triplicate. The standard deviation (SD) values didn’t surpass 5% of the mean values in any experiment. A One-Way ANOVA test was completed to compare the means between different liposome types, and the means between different HDP-2/phospholipid molar ratios within the same liposome group. A p-value < 0.05 was considered statistically significant. Statistical analysis for the AutoDock study was not performed, as no significant variation was observed across the triplicate runs for each docking pair.
Safety Measures and Ethical Issues
Chloroform is toxic at contact with skin and eyes and carcinogenic when ingested. When inhaled methanol is toxic and may cause death when ingested. As the safety precaution, all experiments involving chloroform and methanol were conducted under a certified fume hood. HDP-2 is harmful if reaches a bloodstream. The laboratory coat and safety goggles were worn in the lab. This study has no ethical issues.
Phosphatidylcholine (PC), the most abundant phospholipid in mammalian systems, constitutes up to 80% of total phospholipids in cellular and subcellular membranes [24]. In addition to investigating membranes composed exclusively of CL, PS, SM, or PC, we also analyzed membranes containing PC supplemented with 20 mol% of either CL, PS, or SM. As demonstrated in Table 1, HDP-2 exhibits concentration- dependent hydrolytic activity in mixed phospholipid liposomes, with activity increasing proportionally to enzyme concentration. Among these systems, HDP-2 showed highest activity towards, PC+PS liposomes showed the highest enzymatic activity, whereas it displayed low activity towards PC+SM liposomes displayed the lowest. Conversely, in single-phospholipid liposomes (Table 2), maximal activity of HDP-2 occurred in CL liposomes, whereas, minimal activity was observed in in PC liposomes, In addition, no detectable activity was observed in SM liposomes. The datasets from Tables 1 & 2, graphically represented in Figure 1, reveal that HDP-2 reaches saturation in its activity with phospholipid substrates at a molar ratio of 0.003 (HDP-2/phospholipid, mol/mol). The hydrolytic activity of HDP-2 is determined by both phospholipid type and their membrane packing density [1]. Tightly packed phospholipid bilayers typically hinder phospholipase activity. Liposomes composed solely of non-bilayer cardiolipin (CL) present the most favorable substrate surface for HDP-2 (Figure 1A), while pure phosphatidylserine (PS) membranes offer less optimal conditions for hydrolysis (Figure 1B). Notably, the membrane surface of PC+CL mixtures proves less conducive to HDP-2 activity compared to PC+PS systems (Figures 1C & 1D).
Table 1: Hydrolytic activity of HDP-2 in liposome samples composed of PC + 20 mol% SM, PC + 20 mol% CL, or PC + 20 mol% PS, presented in arbitrary units (a.u.) based on optical density measurements at 550 nm.
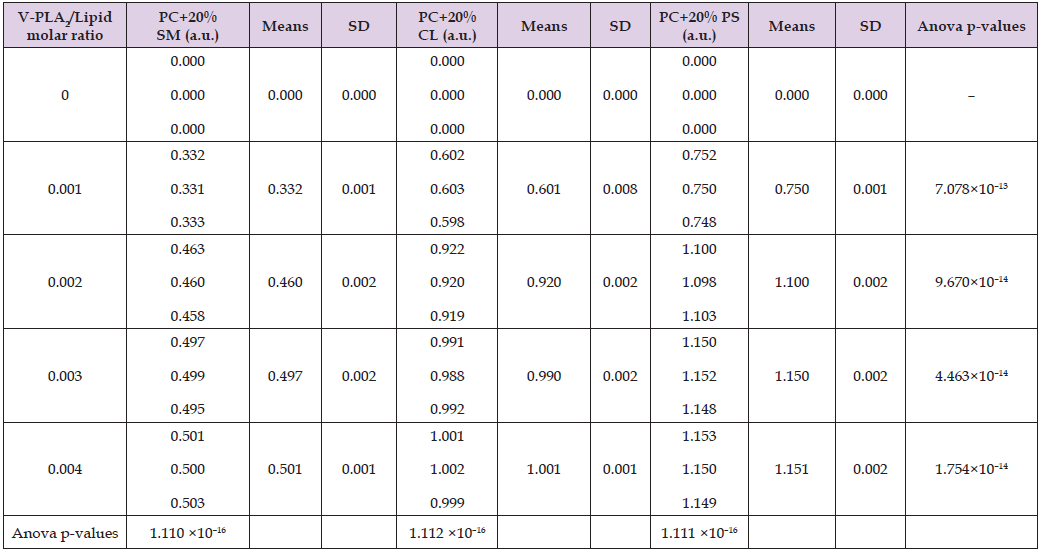
Table 2: Hydrolytic activity of HDP-2 in liposome samples made of SM, PC, CL or PS, presented in arbitrary units (a.u.) based on optical density measurements at 550 nm.
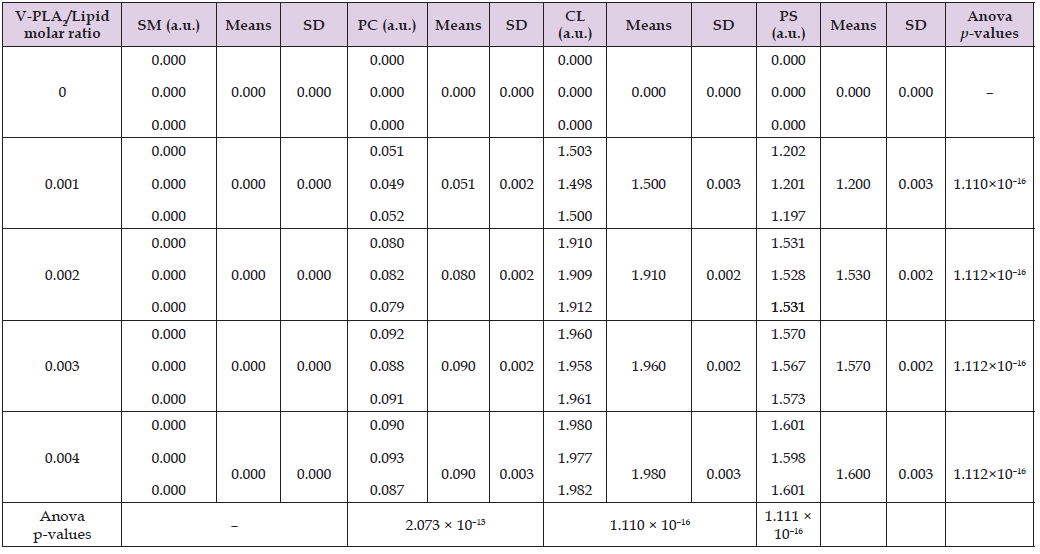
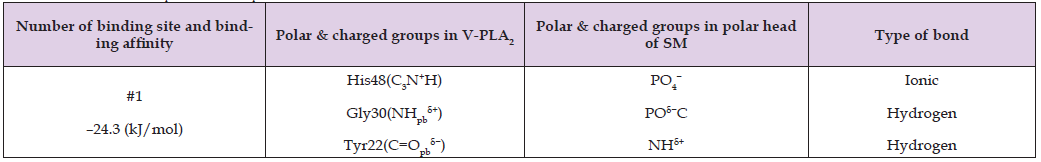
Pure phosphatidylcholine (PC) or sphingomyelin (SM) membranes completely resist HDP-2-mediated hydrolysis (Figures 1F & 1G). In contrast, PC+SM membranes exhibit significantly greater susceptibility to HDP-2 activity than either pure PC or SM alone (Figure 1E). To investigate the molecular mechanisms underlying the hydrolytic activity of HDP-2 in membranes composed of single or mixed phospholipids, we simulated the binding interactions of CL, PS, SM, and PC with the molecular surface of HDP-2 using AutoDock software. The program predicts the nine binding sites with highest binding affinities (expressed in kJ/mol) for each HDP-2-phospholipid pair, where binding affinity quantifies the energy released upon phospholipid binding at specific sites on HDP-2’s molecular surface. The active center of HDP-2 contains critical residues including Gly30, Gly32, His48, Asp49, and Lys69, with Lys69 positioned at the entry to the active site. Our docking simulations revealed distinct binding patterns: for CL, only two of the nine predicted binding sites that involved the active center of HDP-2; for PS, seven sites showed active center binding; while PC and SM each demonstrated four active center binding sites among the nine predicted locations. Tables 3-6 and Figures 2-5 present data for only the highest-affinity binding site (selected from the nine predicted sites) for each HDP-2-phospholipid pair, where the sn-2 bond of the phospholipid shows maximal insertion into HDP-2’s active center.
These tables report the binding site number, binding affinity, specific polar and charged groups participating in intermolecular bonds, and the types of bonds formed. The accompanying figures (Figures 2-5) display only those HDP-2 residues that form hydrogen, polar-ionic, and ionic bonds with the phospholipids. Notably, in the CL-HDP-2 docking pair (Table 3), Asn67 participates in binding despite being outside the active center. Similarly, Tyr22 contributes to binding in both PS-HDP-2 (Table 4) and SM-HDP-2 (Table 6) pairs, although it is not part of the enzyme’s active center. These observations suggest that regions beyond the canonical active site may influence phospholipid binding and orientation. Figure 2 illustrates two alkyl chains of cardiolipin (CL) positioned within the active center of HDP-2, with the sn-2 bond located in the active center. This observation supports experimental findings regarding the HDP-2’s hydrolytic activity in the CL containing liposomes. Figure 3 illustrates the ammonium group of the PS polar head making a polar-ionic bond with Tyr22 within the active center of HDP-2. This interaction facilitates the folding of the PS glycerol moiety, positioning the sn-2 bond deep within the active center of HDP-2. These findings align with experimental data demonstrating the hydrolytic activity of HDP-2 on liposomes containing PS. Figure 4 and Table 5 show that the phosphate group and polar groups of the PC glycerol moiety stabilize the sn-2 bond within the active site of HDP-2.
Table 3: Binding site affinity energy and types of intermolecular bonds between the polar and charged moieties of HDP-2’s amino acid residues and the polar and charged groups of the cardiolipin (CL) polar head, as predicted by AutoDock program simulations. The notations for the polar and charged groups in the CL polar head are provided in Figure 2B. In the notation NHpb δ+, Pb denotes a peptide bond.
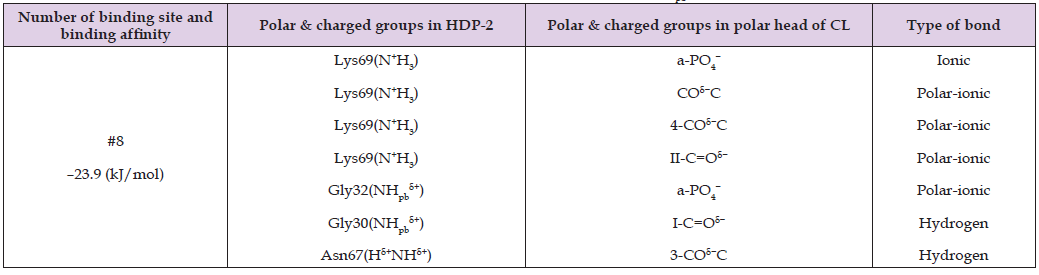
Table 4: Binding site affinity energy and intermolecular bond types between the HDP-2’s polar and charged groups of amino acid residues and the PS polar head’s polar and charged groups, as predicted by AutoDock simulations. The notations for the PS polar head’s polar and charged groups are provided in Figure 3A. In the notations NHpb δ+, pb denotes a peptide bond.
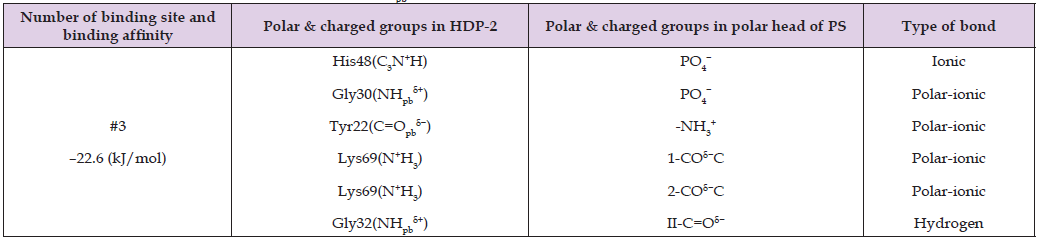
Table 5: Binding site affinity energy and intermolecular bond types between the HDP-2’s polar and charged groups of amino acid residues and the PS polar head’s polar and charged groups, as predicted by AutoDock simulations. The notations for the PS polar head’s polar and charged groups are provided in Figure 3A. In the notations NHpb δ+, pb denotes a peptide bond.
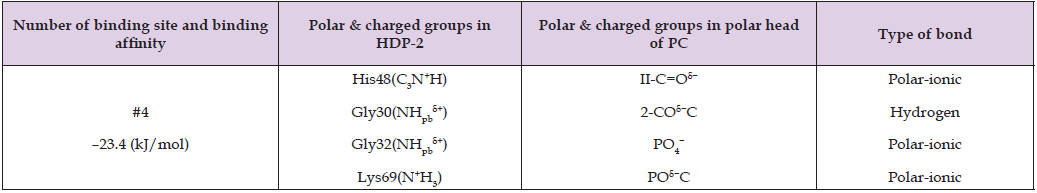
Table 6: Binding site affinity energy and intermolecular bond types between polar and charged groups of HDP-2’s amino acid residues and polar and charged groups of SM polar head predicted by AutoDock program simulation. Polar and charged groups notations for SM are given in Figure 5B. Pb in NHpb δ+ and C=Opb δ− denotes a peptide bond.
This observation aligns with the HDP-2 hydrolytic activity mode in PC+SM liposomes but contrasts with HDP-2 negligible activity in pure PC liposomes. These findings suggest that the tightly packed bilayer structure of pure PC protects phospholipids from hydrolysis, whereas the less compact packing in PC+SM membranes makes the sn-2 bond of PC more accessible for enzymatic hydrolysis. Table 6 and Figure 5 illustrate the stabilization of SM within the peripheral region of the active center of HDP-2, highlighting the interaction via a hydrogen bond between the NHδ⁺ group of the sphingosine backbone and the Tyr22(C=Opbδ⁻) group. Notably, this interaction pulls the SM’s sn-2 bond away from the active center, suggesting that this interaction between SM and HDP-2 is not productive for hydrolytic activity of HDP-2. Given the fact that SM occupies the active center of HDP-2 which does not result in the hydrolysis of the sn-2 ester bond of SM, one may conclude that SM may act as a competitive inhibitor. This conclusion aligns with our experimental observations showing no detectable HDP-2 hydrolytic activity in pure SM liposomes (Figure 1G).
This study utilized the FUJIFILM Wako NEFA kit, which employs a colorimetric assay where colorless coenzyme A turns purple upon acylation by free fatty acids released through PLA₂-mediated phospholipid hydrolysis. This method provides a precise and straightforward approach for quantifying PLA₂ activity, with optical density measurements directly reflecting enzymatic activity levels. Our experimental results revealed several key findings regarding HDP-2’s hydrolytic activity. First, the enzyme demonstrated greater activity in pure cardiolipin (CL) liposomes compared to pure phosphatidylserine (PS) liposomes, while showing the opposite preference in mixed liposome systems, with PC+PS membranes exhibiting higher hydrolysis rates than PC+CL membranes. Secondly, HDP-2 showed minimal activity towards (SM) and phosphatidylcholine (PC) liposomes showed minimal hydrolysis, whereas it displayed significant activity towards PC+SM liposomes displayed significant activity. These observations strongly suggest that HDP-2’s hydrolytic activity depends on both phospholipid species and membrane packing density, with PC+PS membranes proving more favorable for enzymatic activity than PC+CL membranes. Similarly, the packing arrangement in PC+SM membranes appears less dense than in pure PC or SM systems. Complementary AutoDock simulations provided molecular-level insights into these phenomena.
The computational analysis showed productive binding configurations for PS, PC, and CL within HDP-2’s active center, with their sn-2 ester bonds optimally positioned for hydrolysis. In contrast, SM binding occurred peripherally, displacing its sn-2 bond from the catalytic site and resulting in nonproductive interactions that explain its inhibitory effects. The strong correlation between these computational predictions and our experimental hydrolytic activity measurements lends robust support to several conclusions. First, CL and PS serve as readily accessible substrates for HDP-2 across various membrane contexts. Second, PC undergoes hydrolysis primarily in membranes with reduced packing density. Third, SM may act as a competitive inhibitor of HDP-2 activity as supported by data from this study and in our prior publications [13]. The biological implications of these findings are significant given the distinct phospholipid compositions of different cell types. CL’s predominant localization in cardiomyocyte mitochondria, combined with SM’s abundance in neural membranes, suggests that HDP-2 primarily functions as a cardiotoxin through its preferential targeting of cardiomyocytes, with secondary neurotoxic effects resulting from its action on nerve cells. In considering the physiological relevance of these results, the role of PS deserves particular attention. While normally restricted to the inner leaflet of plasma membranes in healthy cells, PS externalization serves as a well-established “eat-me” signal for macrophages during cellular pathology, occurring in processes ranging from apoptosis initiation to viral infection [23,27,28].
Our findings suggest that PS may additionally function as a signal for apoptosis by enhancing PLA₂ activity on neuronal membranes, thereby promoting membrane neurodegeneration. Conversely, SM’s inhibitory effects on PLA₂ hydrolysis may represent an endogenous neuroprotective mechanism against excessive membrane breakdown in nerve cells. The substrate preferences and inhibitory mechanisms of HDP-2 have direct implications for human diseases characterized by dysregulated phospholipid metabolism or membrane integrity. For instance, mitochondrial dysfunction in cardiovascular diseases such as ischemia-reperfusion injury and Barth syndrome involves excessive CL exposure, which may render cardiomyocytes vulnerable to HDP-2-like PLA₂ activity, exacerbating tissue damage [29,30]. Conversely, potential inhibitory role of SM aligns with its neuroprotective function in demyelinating disorders like multiple sclerosis, where SM-rich myelin sheaths resist degradation to maintain neuronal integrity [31]. The observed PS-dependent enhancement of HDP-2 activity further underscores its potential role in pathological apoptosis, as seen in neurodegenerative diseases such as Alzheimer’s and in cancer, where PS externalization facilitates aberrant cell clearance [32,33]. These findings highlight HDP-2 as a model for understanding endogenous PLA₂ enzymes implicated in disease.
Therapeutic strategies could exploit its lipid-specific interactions, such as developing SM-mimetic compounds to inhibit overactive PLA₂ in inflammatory or neurodegenerative conditions. Another approach involves targeting PS externalization with monoclonal antibodies or annexin-based therapies to modulate apoptotic signaling in cancer or autoimmunity [34]. Additionally, engineering CL-stabilizing agents like elamipretide could protect mitochondrial membranes in cardiomyopathies [35]. Future studies should validate these approaches in disease models, particularly those with membrane lipid imbalances, to translate mechanistic insights into clinical applications.
This article has been supported by the start-up grant from the Beijing Chaoyang Kaiwen Academy (E.S.G.) and the NIH grant 5R01NS105783 (R.K.D.). The work pertaining to the preparation of HDP-2 was supported by a grant from the Russian Science Foundation No. 24–15-00280 (Y.N.U.).
Authors have no competing financial and non-financial interests to declare.


