Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nikolai Kocherginsky*
Received: July 20, 2025; Published: August 01, 2025
*Corresponding author: Nikolai Kocherginsky, Next-ChemX, 909 E. Sunnycrest Drive, Urbana, IL 61801, USA; Orcid ID: 0009-0000-9452- 877
DOI: 10.26717/BJSTR.2025.62.009805
New hemodialysis membranes should have minimized immune response and improved blood compatibility. One of polyether sulfone (PES)-based membranes developed with this purpose was covered by a hydrophilic protective layer with uremic metabolites. The paper describing this membrane leads to many questions, some of them are given here.
Keywords: Hemodialysis Membrane; Polyether Sulfone; Protective Polymer Layer; Uremic Metabolites; Zwitterionic Sulfo-Betaine-Based Polymer; Blood Proteins
Previously, in order to minimize blood immune response during hemodialysis, different molecules were immobilized or blended with polyether sulfone (PES) polymers. These additives modified the membrane, enhanced their biocompatibility and also improved the uremic waste dialysis [1,2]. The paper by Dr. Amira Abdelrasoul and co-authors describes a new hemodialysis membrane based on PES [3]. The membrane is covered by a hydrophilic protective layer, which consists of a derivatives of uremic metabolites and a zwitterionic sulfo-betaine- based polymer. Spectroscopy techniques and gravimetry were used to characterize the membrane. Atomic force microscopy, differential scanning calorimetry and zeta-potential measurements were used to characterize the surface properties. In addition, the reduction in platelet factor 4 secretion from the modified membrane compared to the commercial membrane was considered as a demonstration of an improvement of hemocompatibility. The authors used a lot of methods, but the paper also raises a lot of questions, which may be important for a reader.
1. Porosity and the pore size for a dry membrane were determined using a Brunauer-Emmett-Teller (BET) method, and for PES they are 0.06 cm3/g and 1.45 nm. The last number means that the pore size is determined with the accuracy 0.5Å, i.e. less than the hydrogen atom. The result for an average surface roughness is even more “precise”, equal to 52,61nm. Now, on average, the position of atoms is known with accuracy 0.1Å. It is known that BET method is extremely useful as a qualitative guide; but it is not quantitatively correct. Later, based on Figure 13a, the authors claim that the “PES membrane is a microporous structure with pores bigger than 50 nm”, which directly contradicts to 1.45 nm, but still should not be called microporous. According to their patent [4], the pores of modified PES membrane were in a range from 2 to 50 nm.
2. Comparison of the pore volume with the weight in aqueous solutions shows that the membrane is swollen by ~ 20 times. Simultaneously, total water content in PES is only 43% and for modified membranes it is up to 52.4%. Measured by DSC non-freezable water in PES makes only 2.83%, and it is almost 20 times more in modified membranes. Authors believe that “an increased number of hydrogen bonding with moderate energies could result in higher hemocompatibility of membrane material.” Another surprise is that the freezing temperature for non-freezable water in PES is +1.330C (section 3.2.8). By definition, if this water is not freezable, it should be less than 00C.
3. Table 7 in [3] gives calculated energy of water hydrogen bonding. For initial PES the value is -4.79 kcal/mol, and it is - 0.13375 kcal /mol for final sulfobetaine-based polymer. The value is negative and very small, but it is again given with amazingly high precision, 0.01cal/mol. Not a single method can measure this! In comparison, conducted in the same lab simple elevation of patient blood temperature resulted in nearly 32 kcal/mol higher total interaction energy between fibrinogen and polyarylethersulfone [5].
4. Mobility of the system was also used as an index of hemocompatibility. The term mobility was introduced by Einstein to describe diffusion, and it is equal to the diffusion coefficient divided by RT. It has a simple physical meaning of the directed velocity per unit of molar force. Authors use the same term for a different function and should explain it.
This is the explanation: “Mobility of the systems was calculated by the mobility function of water molecules according to Eq. (3):
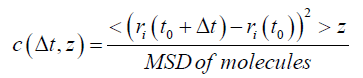
where ri is the location of molecules, and MSD is the mean square displacement.”
Table 6 in [3] gives the values of mobility, and for nonmodified membrane it is 6.96x10-6 (no units are given) and RMV is 1.00x100, which is simply one. List of abbreviations gives only the root mean square, RMS, but it is possible to find relative mobility values (RMC, mobility values of each membrane divided by the mobility values of PES. So, it is not clear if the authors are talking about mobility of membranes or water. Anyway, the modified membrane “owns 45% less mobility in comparison with the PES membrane”.
5. The surface charge of the membrane sheets was measured using a zeta potential analyzer, and it is given in mV instead of coulombs. For example, for PES the surface charge is -6.92 mV, without any comments. Same units are in [4].
6. Talking about role of polar surface layer, the authors believe that the “ring opening of the chosen sultone could induce a more distinct charged nitrogen group”, though the ring structure of propane sultone (CH2)3SO3 does not have any nitrogen!
7. An interesting result could be that “the ex-vivo assessment demonstrated a 16 % reduction in platelet factor 4 secretion for the uremic metabolite-sulfobetaine-modified membrane compared to the control sample, indicating a successful improvement in hemocompatibility.” What was really measured was the desorption of PF4 from different membranes into the patients’ blood. “The measured value for the PES membrane was 414.63 ng/ml” and it decreased after chemical modification. How does it change with time is not known. Again, amazing accuracy and sensitivity! The measurements were repeated three times and standard deviations were calculated but they are not reflected in the paper.
8. The most important test - how fast and efficient was hemodialysis- was not conducted for both nonmodified and modified membranes.
Further a few more examples.
9. Comparison of the titles of papers [3,6] reveals a contradiction and raises a simple question: so, what modification is better, the one which makes the membrane more hydrophilic or more hydrophobic? 10. In the abstract the authors wrote that the zwitterionic layer “was added to the membrane at a rate of 5 to 6 mg/cm2”. Without time I would not call this value the rate.
11. In the introduction: “A patent has recently reported the structure as a successful membrane modifier. Urea, a uremic metabolite, with two nitrogen that could mimic hydrophilic amine sites of biological structures, is used to connect carboxy betaine (CB) to the membrane. The structure is reported to improve the overall hemocompatibility of the dialysis membrane.“ The reference has the name of the inventor and his University in China, but no patent number.
12. Near the end: “In the cases of hydrophilic surface-water molecules interactions, water could possess three distinct types.” The authors are talking about freezing water, non-freezable water, and “intermediate water or freezing bond water”. And further: “Our team’s previous invesitations reflects that a hydrated membrane could be more hemocomapible.”
13. At the end of the paper, the authors write: “Our research aimed is to develop hemocompatible membrane materials with improved properties and demonstrate the efficacy of gadolinium (instead of guanidium?) as a linker for uremic metabolites to enhance membrane hemocompatibility.”
I hope that the PI of this and other similar papers, Associate Professor Amira Abdelrasoul, will be able to give her comments. Recently her name was added to the List of thirteen notable faculty and researchers at the University of Saskatchewan, since the university was founded more than 110 years ago. Our congratulations! Another notable name in this List is professor Gerhard Herzberg, 1970 Nobel Prize in Chemistry, who escaped from Nazi Germany before the Second World War, and then for several years worked at the University of Saskatchewan.


