Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Simon Walz1, Yi Wei2, Moritz Maas1, Wilhelm K Aicher2, Arnulf Stenzl1, Niklas Harland1 and Bastian Amend1*
Received: May 30, 2025; Published: June 09, 2025
*Corresponding author: Bastian Amend, Department of Urology, University Hospital Tuebingen, Hoppe-Seyler-Str. 3, 72076 Tübingen, Germany
DOI: 10.26717/BJSTR.2025.62.009714
Purpose: Recent studies suggested that the efficacy of cancer drugs against carcinoma cells may vary significantly
in two-dimensional cultures versus three-dimensional spheroids. We, therefore, compared the effects
of novel fibroblast growth factor receptor (FGFR)-affecting drugs on three bladder cancer cell lines: T24 with
wild-type (WT) FGFR3 and expressing FGFR3 at low levels, UM-UC-15 inheriting an FGFR3 mutation, and RT112
with an FGFR3 gene amplification and expressing FGFR3 at high levels. The study was conducted in standard
two-dimensional (2D) cultures versus three-dimensional (3D) spheroids.
Methods: The cell lines were expanded in 2D cultures or resuspended in Matrigel® for 3D spheroids. The cells
were incubated with different concentrations of tyrosine kinase inhibitors Erdafitinib®, Lenvatinib®, Cabozantinib
®. Addition of Cisplatin or cultures without drugs served as controls. The cell viability was determined after
24h, 48h, and 72h of incubation by a multimode luminometer technology.
Results: A significant, dose- and time-dependent reduction in cell viability was observed with cisplatin in both
2D and 3D cultures of all lines investigated. In T24, only Erdafitinib® produced robust effects at higher concentrations
in both 2D and 3D assays. In UM-UC-15, Erdafitinib® and Cabozantinib® exhibited cytotoxic effects,
while RT112 appeared to be the least sensitive cell line to Erdafitinib®, Lenvatinib®, and Cabozantinib®.
Conclusion: The sensitivity of bladder cancer cells against active components with a larger molecular weight
seems reduced in spheroids compared to standard 2D cultures. The exact genetic status of FGFR3 must be determined
individually before a therapy decision is coined, as the drug efficacy of such components significantly,
depends on the expression of a suitable target in cells to be targetted.
Keywords: Bladder Cancer; Bladder Cancer Spheroids; Drug Screening; Personalized Medicine
Bladder cancer is one of the most prevalent malignancies worldwide. According to the Global Cancer Observatory (GLOBOCAN), approximately 573,000 new cases and over 212,000 deaths were reported in 2020 [1]. The disease shows a marked gender disparity, being about four times more common in men than in women, and it primarily affects older adults, with the majority of cases diagnosed in individuals over the age of 65 [2]. The most common histological subtype is urothelial carcinoma, also known as transitional cell carcinoma, which accounts for more than 90% of all bladder cancer cases in developed countries [3]. Immune checkpoint inhibitors targeting PD-1/PD-L1 have become standard options for advanced or metastatic disease, particularly in patients who are ineligible for or progress after platinum-based chemotherapy [4]. In addition to immunotherapy- based treatment, advances in molecular biology and genomics have led to the identification of key genetic alterations driving bladder cancer progression, opening new avenues for targeted therapy. Among these, alterations in the fibroblast growth factor receptor 3 (FGFR3) gene have emerged as particularly significant. FGFR3 mutations, fusions, and amplifications are frequently observed in bladder cancer and have been associated with oncogenic signalling that promotes tumour cell proliferation and survival [5]. This has spurred the development of FGFR inhibitors, several of which have shown promising clinical activity in patients with FGFR3-altered tumours [6].
However, the effectiveness of FGFR-targeting therapies can be influenced by the cellular context in which they are tested, particularly the dimensionality of in vitro culture systems [7]. Traditional two-dimensional (2D) monolayer cell cultures have long been the standard in preclinical drug testing. While they offer simplicity and reproducibility as well as cell line-specific assays, these models often fail to recapitulate the complex tumour architecture and microenvironment in vivo with accuracy. In contrast, three-dimensional (3D) spheroid cultures provide a more physiologically relevant model by mimicking key features of solid tumours, including cell-cell and cell-matrix interactions, gradients of nutrients and oxygen, and the development of hypoxic cores [8,9]. As such, 3D models have been increasingly employed to evaluate cancer drug efficacy more reliably [10]. However, the effects of vasculature and enervation on tumour dynamics cannot (yet) be investigated in organoids, spheroids or other 3D tumour models. Recent studies suggest that cancer cells can exhibit markedly different responses to therapeutic agents depending on whether they are cultured in 2D or 3D environments [8,9]. These differences can significantly affect the translational relevance of preclinical findings and even misguide personalized approaches. In this context, evaluating the performance of novel FGFR-targeting drugs across different culture systems and genetic backgrounds is crucial for optimizing predictional models as well as personalized therapeutic strategies.
In the present study, we investigated the effects of novel FGFR-affecting drugs on three human bladder cancer cell lines with distinct FGFR3 profiles: T24 cells, which possess wild-type FGFR3 and express it at low levels; UM-UC-15 cells, which harbour a known activating FGFR3 mutation; and RT112 cells, characterized by FGFR3 gene amplification and high FGFR3 expression. Drug responses were assessed in both 2D monolayer cultures and 3D spheroid models to determine the influence of culture dimensionality on therapeutic efficacy. By comparing responses across these models and genotypes, we aim to gain deeper insight into the relevance of tumor architecture and FGFR3 status in shaping drug sensitivity, with the ultimate goal of informing more effective and personalized treatment strategies for bladder cancer.
Implemented Cell Lines and Profiling for Genetic Alterations
Three bladder cancer (BC) cell lines with distinct features of the FGFR3 were employed to investigate differences in drug sensitivities addressing the FGFR3 pathway. UM-UC15 is a human BC cell line derived from a patient with muscle-invasive disease. The sex and age at sampling of this cell line is not specified [11]. Several mutations but no gene fusion within the FGFR3 and ERBB3 are reported [5,12,13]. The T24 cell line is derived from a female BC patient, who was 82 years old [14]. T24 inherits the FGFR3 wild-type gene. However, mutations have been identified in the HRAS, TP53, and EP300 genes [15,16]. FGFR3 gene fusions are not reported for this cell line. The RT112 cell line is derived from a female BC patient of unknown age [17]. Mutations within the KDM6A gen and fusions within the FGFR3 gene are reported [18,19].
Drug Testing in 2D Cell Cultures
The BC cell lines UM-UC15, T24, and RT112 (ATCC, Manassas, VA, USA) were expanded in culture medium (MEM, complemented by 10% FBS, non-essential amino acids, sodium pyruvate, and antibiotics; all from Fisher Scientific) as requested by the supplier. For the 2D drug tests requiring 2000 cells per 96-well, the cells were harvested using trypsin-EDTA, washed, counted, diluted, resuspended in 100 μL of culture medium, and seeded in flat-bottom 96-well ELISA plates. All 2D drug analyses were conducted in quintuplicates. Following overnight incubation, the culture medium was aspirated and replaced with 100 μL of fresh culture medium supplemented with various concentrations of Cisplatin (ranging from 3 to 30.2 μM), as well as with the tyrosine kinase inhibitors (TKIs) Erdafitinib®, Lenvatinib®, and Cabozantinib® (each ranging from 0.01 to 10 μM). Erdafitinib was used as a specific FGFR inhibitor. Lenvatinib was used as a a multiple kinase inhibitor targeting the three main vascular endothelial growth factor receptors VEGFR1, 2 and 3, platelet-derived growth factor receptor (PDGFR) alpha, c-Kit, the RET proto-oncogene as well as as FGFR 1, 2, 3 and 4. Cabozantinib as a multiple kinase inhibitor without FGFR specificity, targeting VEGFR-2 and c-Met as well as the classical chemotherapeutic agent cisplatin was used as control.
For drug testing, all cell lines were expanded according to the manufacturer’s information. Cells incubated in the culture medium without drugs served as controls. Cells in the culture medium complemented with 1 μL DMSO served as solvent controls. After incubating the cells for 24, 48, or 73 hours with or without the components, the medium was removed, and the cell viability was assessed using Cell- Titer-Glo 2D reagent kits (Promega, Madison, WI, USA) and a GloMax apparatus (Promega, Madison, WI, USA), following the manufacturer’s instructions.
Drug Testing in 3D Cell Cultures
Furthermore, we investigated the effects of Cisplatin, Erdafinitib®, Lenvatinib®, and Cabozantinib® with the same BC cell lines in 3D cultures. Due to some technical limitations of the detection technique and in agreement with the guidelines associated with the reagents and apparatus employed for cytotoxicity analyses, the diameter of the organoids subjected to testing with the CellTiter-Glo 3D chemistry and GloMax apparatus (Promega, Madison, UN, USA) needed to be limited to less than 300 μm. Hence, before cytotoxicity testing, we examined the mean size of the spheroids under a microscope. Subsequently, the spheroids were partially degraded using dispase II (Roche Diagnostics, Penzberg, Germany) for one hour (37 °C, 5% CO2, humidified incubator). The resulting dispersed samples were centrifuged at 150 g for 5 min. at ambient temperature, followed by resuspension in the culture medium. The spheroids were counted and diluted to yield a cell suspension for drug testing, ensuring a concentration of 1000-2000 spheroids per millilitre. Each well was seeded with 100 μL of the spheroid suspension supplemented with 5 μL of Matrigel (BME, R&D Systems, Minneapolis, USA). Next, 1 μL of the drug solution was added to each well to achieve the desired drug concentrations of the active components Cisplatin (ranging from 3 to 30.2 μM) served as positive control, Erdafitinib, Lenvatinib, and Cabozantinib (ranging from 0,01 to 10 μM) as the experimental components. The cells were incubated for 24, 48, and 72 hours. Spheroids cultured in a medium without drugs served as controls, and cultures containing 1% DMSO as solvent controls. All drug analyses on the spheroids were performed in triplicates. The cytotoxicity assay was recorded using the CTG method as mentioned previously.
Data Processing and Statistics
The results obtained from cytotoxicity experiments were processed by exporting the original data to either a spreadsheet software (MS Excel 16.61.1, Microsoft, Albuquerque, NM, USA) or a statistical program (GraphPad Prism 9.4.1., GraphPad Software, La Jolla, CA, USA) for analysis. Mean values of data sets, including quintuplicates of cytotoxicity assays for cells in 2D cultures and triplicates of cytotoxicity assays for 3D organoids, were calculated and presented in the figures as dose-response kinetics. The normalized viability index (NVI) was determined using the formula: NVI = (mean test-blank) / (mean control-blank) × 100, expressed as a percentage (%). To evaluate statistical significance, a two-way ANOVA was performed, and the resulting p-values were summarized as follows: 0.01-0.05 (*) for high significance, 0.0001-0.001 (**) for very high significance, and <0.0001 (***) for extremely high significance. These significant differences were appropriately marked in the artwork.
Cell Lines and Culture Conditions
To evaluate the effect of the FGFR-targeted therapies on bladder cancer in different culture conditions we selected three human BC cell lines with distinct aberrations of the FGFR3 pathway. The tumour cells were treated in 2D and 3D conditions. Before adding the active components to be investigated, culture efficacies and the growth patterns of the adherent cells or cells in spheroids were observed by microscopy. Sufficient numbers of cells for drug treatment over 24 to 72 hours of incubation were corroborated in both, 2D standard cultures as well as 3D spheroids after seeding 2000 cells/well (Figure 1).
Longitudinal Effects of Drug Treatment in 2D and 3D Culture Conditions
Tumor cells were treated at the indicated concentrations for 24, 48, or 72 hours in 2D and 3D cultures. The cell viability was investigated in the three experimental groups employing Erdafitinib®, Lenvatinig ®, and Cabozantinib®, in the concentrations as indicated and compared to the solvent control (Figure 2). To avoid a lack of focus, Figure 2 displays the longitudinal effects of drug treatment in 2D and 3D cultures for the highest and lowest drug concentrations only. As expected, the reduction of cell viability for all evaluated substances was higher for the high concentrations compared to the low drug concentration at all time points (Figure 3). The strongest effect of all drugs on cell viability was observed after 3 days. The most potent effects across all cell lines were observed for the treatment with Cisplatin with a reduction of viability of up to 99% at the highest concentration (2D: T24 97% UM-UC15 95%, RT112 99%; 3D: T24 98%, UM-UC15 92%, RT112 95%). For TKIs, the most potent effects were observed for Erdafitinib with a reduction of cell viability of up to 71% at the highest concentration (2D: T24 46% UM-UC15 52%, RT112 56%; 3D: T24 71%, UM-UC15 33%, RT112 5%). The cell line T24 showed the highest sensitivity to TKI treatment with a reduction of viability of up to 81% at the highest concentration for Erdafitinib®, 54% for Lenvatinib® and 25% for Cabozantinib®. The comparison of 2D and 3d cultures on the drug efficiency showed only for the cell line T24 under Ertafinib® treatment a stronger effect on cell viability in the 3D culture than in the 2D culture (46% compared to 71% viability reduction). Of note, for all other substances and time points the effects of drugs on cell viability were significantly stronger in the 2D culture conditions than in the 3D conditions. This is also reflected in the IC50 values (Table 1). IC50 values were up to 2.8, 1.3x108, 1.9 and 812 times lower for the 2D culture conditions compared to the 3D culture at day 3 for Cisplatin, Erdafitinib®, Lenvatinib® and Cabozantinib ®, respectively.
Table 1: Comparing the impact of drugs on bladder cancer cells in two-dimensional and three-dimensional cell cultures. Displayed is the growth inhibition of the corresponding drug by determining its half maximal inhibitory concentration (IC50) in μM towards cells of analogous bladder cancer cell lines.
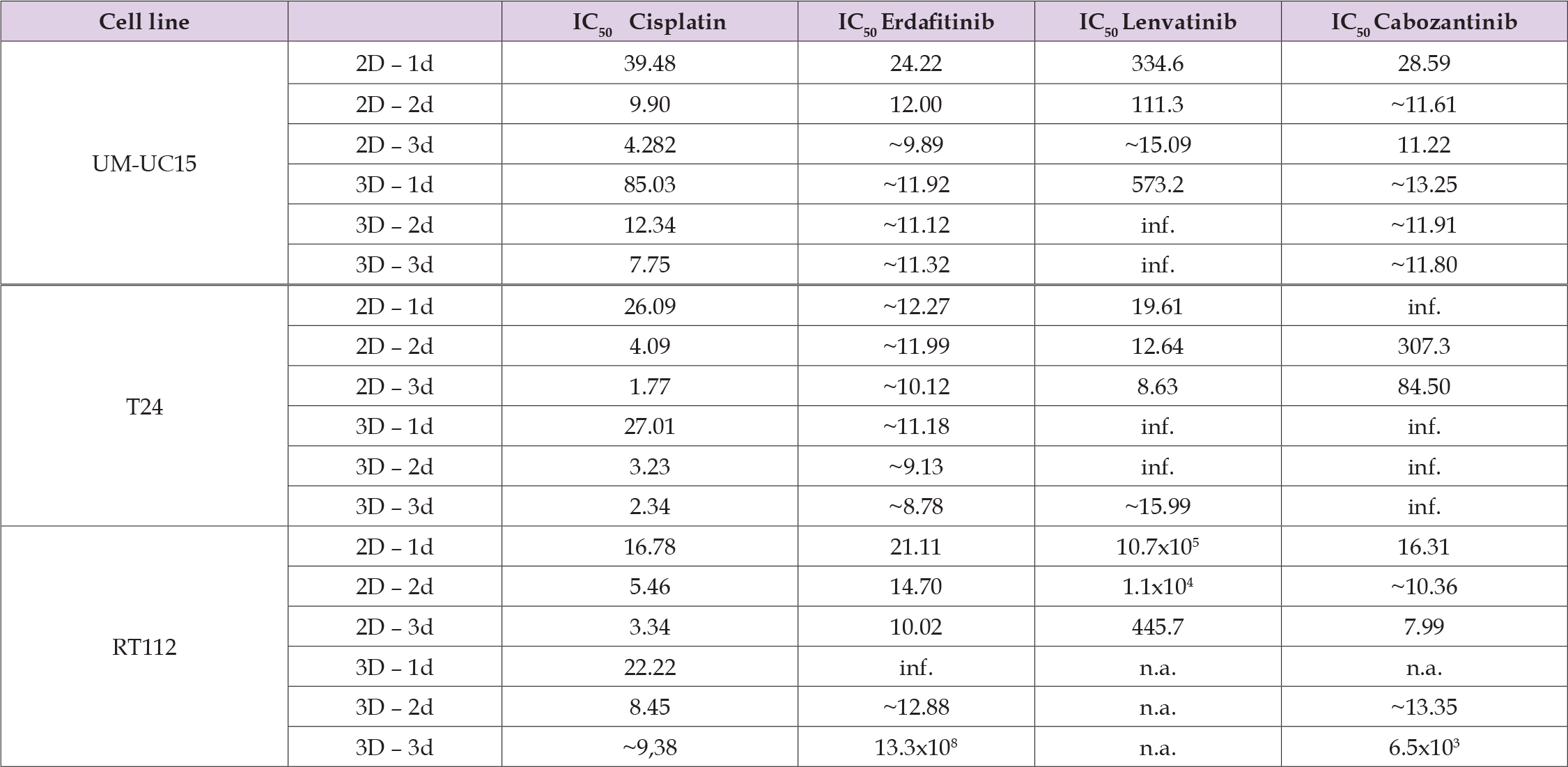
Note: Abbreviations: IC50, half maximal inhibitory concentration; 2D, two-dimensional; 3D, tree-dimensional; d, days of incubation with the respective drug; inf., infinity; n.a., not assasable.
Effect of Drug Concentrations in 2D and 3D Culture Conditions
As outlined above, the most pronounced effects of the drugs on 2D and 3D culture conditions were observed on day 3. Thus we directly compared the effects of the different substances in increasing concentrations at day 3 in 2D and 3D culture conditions. The strongest effects on cell viability for all cell lines and culture conditions were observed at the highest concentrations of each drug. Again Cisplatin treatment had the strongest effect on cell viability for all three cell lines. Differences between 2D and 3D culture for Cisplatin were observed for the two lower drug concentrations, where the reduction of cell viability was significantly increased for the 2D culture condition. Among the TKIs employed in this study, Erdafitinib® yielded the most pronounced effect. For all TKIs and cell lines, despite T24 under Erdafitinib®, the effect on cell viability was stronger in the 2D culture than in 3D culture. The observed difference between 2D and 3D culture was most obvious in the highest drug concentration for all TKIs. Together, this data underscores the importance of evaluating drug effects under different culture conditions and validates existing reports that indicate that doses and drug effects significantly vary between 2D and 3D cultures.
The results of this study present a comprehensive evaluation of the effects of FGFR-targeted therapies on bladder cancer cell lines under varying culture conditions, specifically contrasting two-dimensional (2D) and three-dimensional (3D) environments. The findings indicate that the efficacy of these therapies is influenced significantly by the dimensionality of cell culture as described before for other tumours [20]. This emphasizes the importance of appropriate models in preclinical research, which mimic the features of the corresponding tumour comprehensively [8]. The selection of three distinct human BC cell lines, expressing a wildtype FGFR3 or harbouring aberrations of the FGFR3 pathway as described in this tumour, provided a robust framework for assessing the effects of targeted therapies in vitro. The successful establishment of both 2D and 3D cultures, confirmed by microscopy, ensured that the experimental design reflected varying tumour architectures and phenotypes, which is critical for the comparability of the in vitro analyses with the clinical situation and future translational relevance [8]. The longitudinal analysis of cell viability in both culture conditions revealed a consistent trend: as expected, higher concentrations of drugs lead to a greater reduction in cell viability across nearly all time points. These findings were in line with established pharmacological principles in BC cell lines, affirming that dose-response relationships are maintained in both 2D and 3D cultures [21,22].
As previously mentioned, Cisplatin, in clinical situations well established as a cytotoxic agent, resulted in up to a 99% reduction in cell viability at elevated concentrations, confirming itself as the most effective drug in this experiment, regardless of the cultivation method used, whether in 2D or 3D. This finding aligns with existing literature that highlights Cisplatin’s potency in treating bladder cancer [21]. Therefore, Cisplatin continues to play a guideline-recommended therapeutic key component both in muscle-invasive and metastatic BC provided that the patient is eligible [23]. Interestingly, the differences in drug efficacy between 2D and 3D cultures are particularly pronounced for the TKIs studied. Erdafitinib® demonstrated the highest efficacy among the targeted therapies, with a notable reduction in cell viability in both culture conditions. The observation that T24 cells exhibited the highest sensitivity to Erdafitinib® treatment suggests that this BC line expressing the wildtype FGFR3 retained the highest sensitivity to Erdafitinib® and Lenvatinib®, but not to Cabozantinib®. This correlation between wildtype FGF-receptor expression and a selective TKI sensitivity underlines the importance of interindividual drug screens for individual cancer patients to grant sufficient drug efficacy early on. This difference in cytotoxic efficacy in 2D versus 3D in vitro systems possibly applies to other TKIs in the research pipes as well.
At present, the molecular or structural differences among the TKIs applied in correlation to their efficacy on cells with or without FGFR3 mutations are not fully explored, neither in 2D nor in 3D cell culture systems. Moreover, intracellular signalling pathways linking the engagement of the FGFRs to the cell nucleus, have to be taken into consideration as well. However, such experiments were not the focus of this study and thus remain to be investigated in the future. Concerning the culture dimensionality, the results clearly indicate that the 2D culture conditions generally yield stronger drug effects compared to 3D conditions for most treatments. Limited diffusion of the drugs in 3D constructs is one point in this context. In addition, this discrepancy may be attributed to the differences in cell-cell and cell-matrix interactions that occur in 3D cultures, which can alter drug penetration and cellular responses as well. These findings are in line with reported higher drug resistance levels in 3D cultured lung cancer cell lines compared the their corresponding 2D model [24]. The finding that Erdafitinib had a greater effect in 3D cultures specifically for T24 cells may suggest unique mechanisms of action or enhanced drug uptake that merit further investigation. Moreover, the IC50 values presented support the conclusion that drug efficacy is significantly affected by the culture conditions, with 2D cultures exhibiting lower IC50 values for cisplatin and TKIs. These results emphasize the necessity for careful consideration of the experimental model used when assessing drug efficacy, as the differences observed could influence therapeutic decision-making in personalized drug screening aiming for personalized medicine.
In summary, this study provides compelling evidence that the effects of FGFR-targeted therapies on bladder cancer cell lines are markedly influenced by the dimensionality of the culture system employed. The pronounced variations in drug efficacy between 2D and 3D models highlight the importance to complement stndard 2D drug efficacy studies by 3D culture systems to better mimic the tumour microenvironment. Future studies should aim to elucidate the underlying mechanisms responsible for these differences, which could ultimately inform the development of more effective treatment strategies for bladder cancer. The validation of these findings in clinical settings will be essential for translating these preclinical observations into therapeutic advancements.
Conceptualization: N.H., B.A., W.K.A and A.S.; methodology: S.W., Y.W., N.H. and W.K.A; validation: N.H., S.W. and W.K.A.; formal analysis: Y.W. and S.W.; investigation: Y.W., S.W., M.M.; data curation: Y.W., S.W., N.H., W.K.A; writing—original draft preparation, S.W.,M.M., W.K.A.; writing—review and editing, all authors.; visualization: Y.W., S.W.; supervision, S.W., A.S.; funding acquisition: A.S.; All authors have read and agreed to the published version of the manuscript.
This research was funded in part by grants from the DFG to A.S. (GRK2543 # 409474577 and, from the EU (OCT-detector E!115301/01QE2133C) respectively, the Urologie e.V. Tuebingen, and in part by institutional funds.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Tuebingen Hospital (file # 804/2020/B02).
Informed consent was obtained from all subjects involved in the study according to the regulations of the Ethics Committee of University of Tuebingen Hospital.
The data of this study will be made available to all colleagues from public institution dedicated only to research and education upon justified request.
The authors declare no conflict of interest for this study.


