Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mohammed A Jamali1,2*, Suad M Abdeen2 and Thazhumpal C Mathew3
Received: April 03, 2025; Published: April 24, 2025
*Corresponding author: Mohammed A Jamali, Department of Psychiatry, College of Medicine, Health Sciences Center, Kuwait University, Kuwait
DOI: 10.26717/BJSTR.2025.61.009610
Objective: To assess the effect of vitamin-D receptor (VDR) gene (located on chromosome 12q13.11) polymorphismsFok1
(rs2228570) and Apa1(rs7975323), and Vitamin D deficiency on the risk of developing obesity and
Type 2 Diabetes (T2D) among young adults in Kuwait.
Material and Methods: A cross-sectional study that included 201 medical and dental students in Kuwait University
(Males = 99; Females = 102). Using both logistic and linear regression models, we analyzed the VDR gene
polymorphisms Fok1 (rs2228570) and Apa1 (rs7975323) association on obesity using body mass index (BMI)
as a binary categorical variable (non-obese, BMI<25 vs. obese, BMI>25), as well as BMI as continuous variable.
Apa1 and Fok1 association with Family History (FH) of T2D was determined using binary logistic regression. All
regression analysis used both additive and dominant genetic models, adjusted for age and gender. Genotyping
was performed using TaqMan® SNP Genotyping Assay, and ABI 7500 fast real-time PCR system SDS software
used for allelic discrimination.
Results: Fok1 and Apa1 were not significant predictors of obesity (p > 0.05) when analyzed BMI as a continuous
variable as well as categorical variable, in both dominant and additive models, after adjusting for both age
and gender. In addition, Fok1 and Apa1 were not significant risk factors for T2D in both adjusted dominant and
additive model. (p > 0.05). Fok1 allele frequency was (G: 75.6%; A: 24.4%) and did not significantly differ from
the global allele frequency (p-value > 0.05); however, the VDR Apa1 allele frequency was (A: 69%; C: 31%; p-value
<0.001). As for genotype frequencies distribution, Fok1 (AG: 37.8%; AA: 5.5%; GG: 56.7%), and Apa1 (AC:
32.8%; AA: 52.2%; CC: 15%) were not consistent with the Hardy-Weinberg Equation.
Conclusion: There was no significant effect of Fok1 (rs2228570) or Apa1 (rs7975232) on obesity or T2D in
healthy young adults of Kuwait, using both additive and dominant genetic models.
Keywords: Apa1; rs2228570; Fok1; rs7975232; Single Nucleotide Polymorphisms; SNP; Vitamin D Deficiency; Hypovitaminosis D; Obesity; Body Mass Index; BMI; Type 2 Diabetes; T2D
Abbreviations: MKH: Mubarak Al-Kabeer Hospital; CBC: Complete Blood Count; SNPs:Single Nucleotide Polymorphisms; GPA: Grade Point Average; TG: Triacylglycerol; FBS: Fasting Blood Sugar ALT: Alanine Aminotransferase; TSH: Thyroid-Stimulating Hormone; AST: Aspartate Aminotransferase
The number of young adults with obesity and Type 2 Diabetes (T2D) is increasing, posing a health challenge in Kuwait. T2Dis a multifactorial condition driven by genetic predisposition, environmental and lifestyle-related factors (Kueh, et al. [1]). T2D affects 25.4% of Kuwait’s residents between 30-60 years, has led to rising healthcare costs due to both the increasing number of cases and the associated expenses (Channanath, et al. [2]). Family members often develop obesity together because genetic factors strongly influence this risk factor for T2D (Lima, et al. [3]). According to the thrifty gene hypothesis, human bodies are efficient at conserving energy, makinghumans prone to gaining weight when food is plentiful (Gupta, et al. [4]). Genetic variations in the vitamin D receptor (VDR) gene (located on chromosome 12q13.11), particularly single nucleotide polymorphisms (SNPs), which eventually leads to metabolic dysfunction (Abouzid, et al. [5]). Vitamin D plays a crucial role in calcium homeostasis and insulin regulation which enhance metabolic health. Its active metabolite, calcitriol (1,25-dihydroxy vitamin D), binds to VDRs expressed in insulin-secreting pancreatic beta-cells, modulating calcium influx, insulin secretion, and sensitivity (Bikle, et al. [6]). Vitamin D deficiency, influenced by limited sunlight exposure, dietary insufficiency, and genetic factors, is highly prevalent in Kuwait with 56% of adults having insufficiency and 27% deficiency (Zhang, et al. [7]). Vitamin D deficiency is strongly associated with increased risks of obesity, T2D, and cardiovascular disease (Paschou, et al. [8]).
Genetic polymorphisms in the VDR gene (located on chromosome 12q13.11), such as Fok1 (rs2228570, exon 2 C>T) and Apa1 (rs7975232, intron 8 G>T), have been linked to metabolic disorders (Maepa, et al. [9]). The Fok1 polymorphism is associated with dyslipidemia, particularly elevated low-density lipoprotein cholesterol (LDL-C), while the Apa1 polymorphism has been associated to insulin resistance and metabolic syndrome in specific populations (Karam, et al. [10]). The literature reported varied association of obesity and Vitamin D receptor (VDR) gene. In one study, obesity was not directly associated to the VDR gene polymorphism Fok1 (Akter, et al. [11]). However, Jia J et al found that the (TT) genotype was associated with increased LDL-C levels, promoting dyslipidaemia (Jia, et al. [12]). The GG genotype of the Vitamin D receptor (VDR) gene polymorphism Apa1was associated with insulin resistance in Asians (Hanetal,[13]). In middle-aged Russian women, AA genotypes of rs7975232 were substantially linked with metabolic syndrome characteristics (Karonova, et al. [14]). The diversity and variability of gene and obesity in literature is mixed depending upon region or population. However, findings remain inconsistent across populations, emphasizing the need for population-specific studies. Research has identified a significant association between the Fok1 GG-genotype and T2D in Middle Eastern populations, while meta-analyses suggest its relevance is more pronounced in Asian populations (Totonchi, et al. [15]). Conversely, no definitive association has been established between Apa1 and T2D in global studies.
Existing studies provide mixed evidence on the association of these polymorphisms with obesity and T2D, emphasizing the need for population-specific research. This study aims to investigate the effect of Fok1 and Apa1 polymorphisms, along with vitamin D deficiency, on obesity and T2D in young adults in Kuwait, in order to understand these interactions and provide valuable insights on Kuwaiti young population.
Hypothesis
H0: There is no association between Apa1, Fok1 gene polymorphisms and vitamin D deficiency on the risk of developing obesity and T2D risk in healthy young adults of Kuwait.
HA: There is an association between Apa1, Fok1 gene polymorphisms and vitamin D deficiency on the risk of developing obesity and T2D risk in healthy young adults of Kuwait
Objectives
To assess the effect of VDR gene polymorphisms Fok1(rs2228570), Apa1(rs7975323), and vitamin D deficiency on the risk of developing obesity and T2D in young adults with family history of T2D.
Study Design
A cross-sectional study was conducted including 201 Kuwait University medical and dental students. The study was approved by the Joint Research Ethical Committee of the Ministry of Health of Kuwait and Kuwait University’s Faculty of Medicine. The committee evaluated and approved Arabic and English parental consent forms for minors under 21 years of age and adult consent forms. This research was supported by the Kuwait University College of Graduate Studies and awarded as part of a Master’s Degree Thesis (0545598). The thesis is available at National Library of Kuwait (ISBN: 978-99906- 1-546-3). The consent papers clearly stated the project’s goals, risks, and benefits, the blood collection technique, and members’ rights to data confidentiality and to exit the study at any time. As requested by the Ethical Committee, the consent form stated that all blood samples were discarded after lab examination and would not be used for future study.
Samplerecruitment and Sampling Technique
The total sample size consisted of 201 healthy students, including 99 males and 102 females. Phenotypically healthy medical and dentistry students without visible disability at Kuwait University, who agreed to provide a blood sample, answer the questionnaire, undergo anthropometric measurements, and sign the consent forms, were included in this study. The students were randomly and anonymously selected using the class list, regardless of their year of admission, grade point average (GPA), nationality, or age. We excluded students in the 1st and 7th academic years due to their difficult accessibility. Additionally, authors excluded academic staff, participants who are less than 21 years old without parental consent, as well as those who agreed to complete the questionnaire but declined to give a blood sample, and vice versa.
Clinical Assessment and Anthropometric Parameters
The data collection started in the Pathology Department at the Faculty of Medicine, acquiring a signed adult’s or parental consent form each participant. The participants then answered a 2-minute questionnaire with 13 questions (Appendix), designed to collect demographic and clinical information. The questionnaire included the following variables: student serial number, age, gender, GPA in the foundation year, and whether the participant had diagnosed with medical conditions such as diabetes mellitus, hypertension, or dyslipidemia. The presence of positive family history of diabetes mellitus and hypertension was also recorded. Additional variables included smoking status, number of packs smoked per day, and number of exercise days per week. A blood pressure reading was obtained after sitting and resting in a private room for 10 minutes as a standardization method. An anthropometric measurement, including waist circumference, weight, and height, were obtained afterwards.
Blood Collection and Storage
After signing the consent form, answering the questionnaire, completing the anthropometric parameters, certified and licensed phlebotomists from Mubarak Al-Kabeer Hospital (MKH) drew 12cc of blood from each participant. Blood samples were immediately labelled with serial numbers matching the labels on corresponding questionnaires to protect participants’ identity and confidentiality. The samples were temporarily stored in an icebox full of ice until transferred to Mubarak al-Kabeer Hospital (MKH) laboratory for same day analysis. Lavender cap vacutainers were sent for Complete Blood Count (CBC), and the remaining blood sample was retained for DNA Extraction and SNP Genotyping. Yellow cap vacutainers were retained for serum analysis of multiple biochemical variables. The remaining lavender blood samples were sent back in icebox directly to the Pathology Department Laboratory at the Faculty of Medicine, where they were stored in -20 °C freezer for DNA extraction and genotyping assays for a later stage.
Biochemical Analysis of Serum Samples
Total cholesterol, HDL-cholesterol, triacylglycerol (TG), fasting blood sugar (FBS), urea, creatinine, albumin, iron, aspartate aminotransferase (AST), alanine aminotransferase (ALT), free thyroxine (T4), thyroid-stimulating hormone (TSH), and cortisol levels were measured using an automated analyzer (DXC 800; Beckman Coulter, Brea, CA, USA). LDL-cholesterol was calculated using the following formula (LDL = (0.97 × TC)-(0.93 × HDL)-(0.19 × TG) (Molavi, et al., 2020). Apolipoprotein B (Apo B), and Apolipoprotein A1 (Apo A1) were analyzed by protein chemistry analyzer (IMMAGE 800; Beckman Coulter, Brea, CA, USA). Plasma 25(OH) Vitamin D concentration were measured by electrochemiluminescence (ECL) immunoassay (Cobas e 411 Analyzer; F. Hoffmann-La Roche AG, Basel, Switzerland). Hemoglobin (Hb), white blood cells (WBC), and platelets counts were obtained using the hematology analyzer (LH 750; Beckman Coulter, Brea, CA, USA).
DNA Extraction
Whole blood was collected in EDTA-containing vacutainers, and genomic DNA was extracted from leukocytes using QIAamp DNA Mini Kit (Qiagen, CA, USA). An aliquot of a whole blood sample (200 μL), 20 μL proteinase K to digest proteins, and 200 μL Lysis buffer AL were all mixed in the centrifuge tube. The mixture was incubated in a water bath at 56 °C for 30 minutes. Next,200 μL ethanol was added to precipitate remaining protein in suspension, followed by a brief spin. The mixture was then applied to QIAamp Mini Spin Column and centrifuged. DNA was adsorbed onto QIAamp silica membrane, and subsequent washing with 500 μL of each AW1 and AW2 buffers ensured complete removal of residual contaminants without affecting DNA binding. Finally, 100 μL of elution buffer AE was added to elute the purified DNA in a concentrated form. The DNA quantity for each sample was determined by measuring the optical density at 260 nm wavelength using a spectrophotometer (ThermosScientific Nanodrop 8000).
SNP Genotyping
Genotyping of VDR gene polymorphisms (rs2228570 and rs7975232) was performed using TaqMan® SNP Genotyping Assay (Applied Biosystems, Foster City, USA), which included a predesigned mix of unlabeled polymerase chain reaction (PCR) primers and the TaqMan® minor groove binding (MGB) probes (FAM™ and VIC® dye-labeled). The primers and probes used for both genesare mentioned in Table 1.
DNA Extraction and Genotyping
DNA was extracted from 200 μL of whole blood collected in EDTA- containing vacutainers using QIAamp DNA MiniKit (Qiagen, CA, USA) (Tagliaferro, et al. [16]). Genotyping of the Apa1 and Fok1 gene polymorphism was performed using TaqMan®SNP Genotyping Assay (Applied Biosystems, Foster City, USA) (Tie, et al. [17]). The ABI 7500 Fast Real-Time PCR system and SDS Software (Life Technologies, CA, USA) was used to perform and analyze the allelic discrimination (Jamali, et al. [18]). All TaqMan® SNP Genotyping Assays were designed to work with TaqMan® Universal PCR Master Mix. The SNP genotyping procedure used an Allelic Discrimination (AD) assay, a multiplexed (more than one primer and probe paired per reaction) endpoint assay (at the end of the PCR process data was collected) that detects variants of a single nucleic acid sequence. The AD assay classified unknown samples as: Homozygotes (samples having only alleles 1 or alleles 2), or Heterozygotes (samples having both allele 1 and allele 2). The AD experiment performs thermal cycling which involves 5 stages: a pre-read run, or initial denaturation at 95 °C for 5 min, followed by an amplification run involving 32 cycles of denaturation at 94 °C for 30 sec, annealing at 59 °C for 30 sec, and extension at 72 °C for 30 seconds. Lastly a post-run, or a final extension step at 72 °C for 5 min. The thermal cycling was performed separately for each SNP genotype in a 20 μL reaction mixture volume containing 3 μL of template DNA, 1.5 μL for each forward and reverse primers, 3 μL of dNTP mix, 2 μL of PCR buffer (10X), 1.2 μL MgCl2, 0.2μLTaqDNAPolymerase with concentration of 5 U/μL, and 7.6 μL Nuclease-free water. The thermal cycling was performed using the Applied Biosystems 7500 Fast Real-Time PCR System (life technologies, CA, USA) which uses fluorescent-based PCR chemistries. Data analysis was performed using 7500 Fast SDS Software (Life technologies, CA, USA), after creating the AQ Plate Document.
Statistical Analysis
Data entry and analysis were completed using SPSS software (version 25; SPSS Inc, Chicago, IL, USA). All continuous variables were presented as mean, and standard deviation (±SD) while categorical variables were expressed as count (n)and percentage (%). BMI (Body Mass Index) was categorized according to the National Institutes of Health classification: underweight ≤ 18.5, normal weight = 18.5-24.9, overweight = 25.0-29.9, obesity ≥ 30.0(Kassahun, et al. [19]). Vitamin D status was categorized as: optimal ≥75 (nmol/L), insufficiency = 50.1-75 (nmol/L), mild deficiency = 25.1-50 (nmol/L), severe deficiency ≤ 25 (nmol/L) (Nahwegahbow, et al. [20]). The metabolic syndrome was classified according to the International Diabetes Federation (IDF) criteria (Nwankwo, et al. [21]). Shapiro-Wilk test was used to assess normality for all variables. The normally distributed variables such as height, HDL and Hb were tested using independent samples t-test, ANOVA and other non-normally distributed variables were test used non-parametric tests. P-values for continuous variables were calculated using parametric Independent-Samples t-test and ANOVA test for Height, HDL, and Hb (Normally distributed).
P-values for the remaining variables were generated by non-parametric Mann-Whitney U test and Kruskal-Wallis H test (non-normally distributed). Pearson’s chi-square test and Fisher’s exact test (only if the expected count is less than < 0.05) were used to determine significant differences between genotype and allelic frequencies in our study to global frequencies obtained from the 1000Genomes study, using Hardy-Weinberg Equation (Consortium [22]). Linear regression models were used to predict factors associated with BMI and Vitamin D levels, represented by adjusted β-coefficient and 95% Confidence Interval (CI). Binary Logistic regression models were used to predict factors associated with obesity (Non-Obese vs. Obese) and T2D, presented as adjusted OR and 95% CI. P-value <0.05 was considered statistically significant.
In this study,201 students, including 99 males and 102 females, with mean age of 21.0+ 2.0 years and mean BMI of 25.35 ± 4.79 (Kg / m2). The BMI, obesity, vitamin D status, and vitamin D levels showed statistically significant difference between males and females, with majority of males 59 (59.6%) were obese as compared to females 33 (32.4%). Additionally, vitamin D deficiency was most commonly reported among 89 (89.9%) males than 82 (80.4%) females. Furthermore, females (55.9%) had more positive family history of T2D than males (44.4%), although this difference between groups was not statistically important (p = 0.105; Table 2). While determining relation of Fok1 and Apa1 genotypes with, obesity, FH of T2D, vitamin D status, and deficiency, there is no statistically significant relation found (p > 0.05; Table 3). The Fok1 and Apa1 genotypes did not relate to Body Mass Index categories or vitamin D status as tested by the Kruskal- Wallis H test (Fok1 p=0.237, Apa1 p=0.459). The Fok1 GG genotype appeared most often in the normal BMI group (56.1%) while the Apa1 AA genotype dominated both normal (47.6%) and overweight (34.3%) groups. The severe vitamin D deficiency group contained 45.6% participants with Fok1 GG and 44.8% participants with Apa1 AA genotypes. The results show that genetic variation does not affect body weight status or vitamin D level groups (Table 4).
Note: Chi-square was used to generate p-values (indicated by ‘*’ asterisk sign) for categorical variables, while remaining p-values were generated by non-parametric Mann-Whitney U test for continuous variables. BMI = Body Mass Index, FH of T2D = Family History of Type 2 Diabetes. “Significant p-values were bold”
Note: P-value was generated non-parametric Kruskal-Wallis H test for Mean BMI and Vitamin D levels, whereas Chi-square test was used for the other variables, indicated by ‘*’ asterisk sign.
Note: P-value was generated using non-parametric Kruskal-Wallis H test. Shapiro-Wilk test was used to assess normality (not shown).
Fok1 and Apa1 SNPs showed significant difference between our sample data and the global 1000 Genomes study. Fok1 genotype analysis demonstrated significant differences compared to global data (p = 0.028), with 56.7% participants displaying GG, compared to an expected 45%. However, allele frequencies for Fok1(G: 75.6%, A:24.4%) were not significantly different from global expected figured (G: 67%, A: 33%, p = 0.077). In contrast, both the genotype and allele frequencies for Apa1 differed significantly (p < 0.001). The AA genotype observed in 52.2% participants, compared to an expected26.4%, while the A allele frequency was 69%, compared to 51.5% globally. These findings indicated that Apa1 polymorphisms exhibited distinct regional variations, but Fok1 allele patterns are consistent with global patterns (Table 5).
Table 5: Comparison of Apa1, Fok1genotypeand allele frequencies to global frequencies obtained from the 1000 Genomes study.
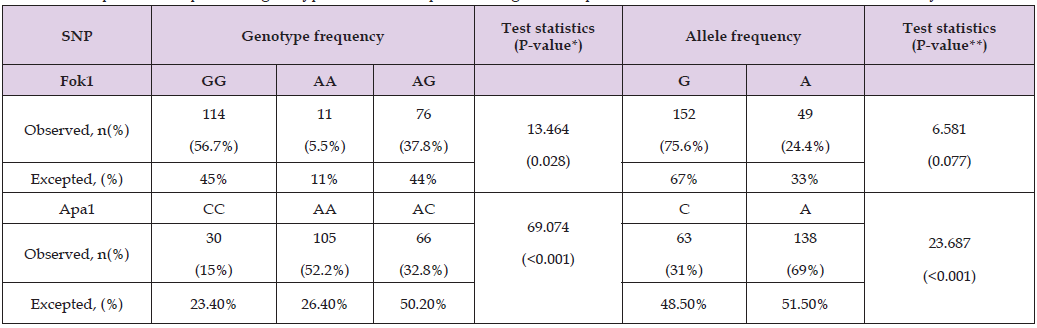
Note: P-value was generated using non-parametric Kruskal-Wallis H test. Shapiro-Wilk test was used to assess normality (not shown).
In Table 6, linear regression model was performed to determine the predictors of vitamin D levels, neither Fok1 nor Apa1 SNPs were significant predictors of vitamin D levels in this study population, even after adjusted for age and gender (Fok1 p = 0.85, Apa1 p = 0.76; model 3). Age showed a marginal association with vitamin D levels in the unadjusted model (β = 0.13, p = 0.07). Therefore, Fok1 and Apa1 SNPsplus age and gender were not associated with vitamin D levels. Fok1 and Apa1 SNPs were not statistically significant predictors of linear BMI or categorical BMI (p > 0.05) determined using linear and logistic regression analysis. For Fok1, GG genotype insignificant association for continuous BMI (β-coefficient: 0.40, 95% CI: -0.452 to 1.260; p = 0.35) and for categorical BMI (OR =1.18, 95% CI: 0.801 to 1.750; p = 0.39). Similarly, for Apa1, the CC genotype (p = 0.35) and GG genotype showed no consistent association between continuous and categorical BMI(β=0.40, 95% CI 0.452 to 1.260, p=0.35; OR=1.18, 95% CI 0.801 to 1.750, p=0.39) respectively. Therefore, Fok1 and Apa1 SNPs were not the predictors of BMI either continuous or categorical (Table 7).
Note: Model 1: adjusted for age only.
Model 2: adjusted for gender only.
Model 3: adjusted for both gender and age.
‘-’indicated empty cells
Table 7: Linear and Logistic regression models to predict factors associated with linear BMI and categorical BMI.
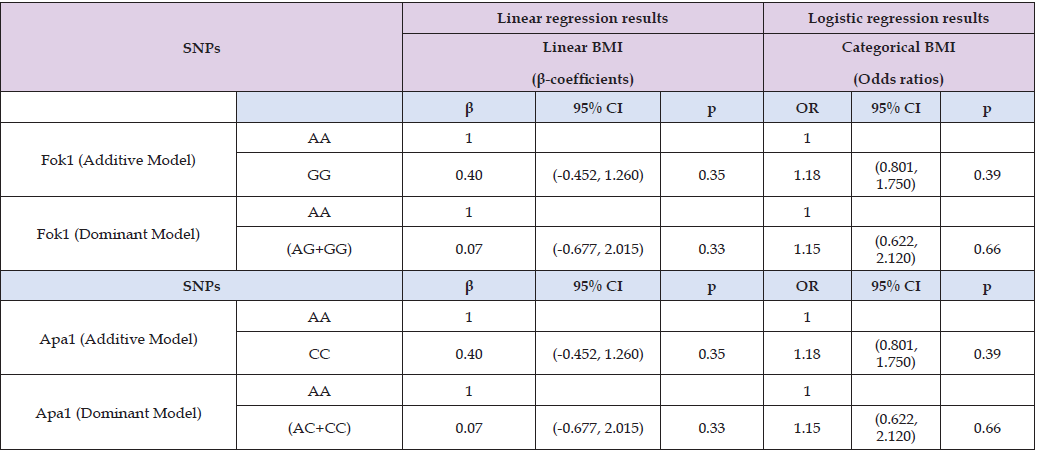
Note: Additive model = (XX vs. XY vs. YY), dominant model = (XX vs. XY+YY).
Obesity (dependant binary outcome) was defined as: Non-Obese = (BMI <25) and Obese = (BMI =>25).
Additive and dominant models are adjusted for both gender and age. P-value <0.05 is considered statistically significant
In Table 8, binary logistic regression model demonstrated that Fok1 SNP was a statistically significant predictor of obesity across unadjusted and adjusted models for age and gender (OR = 1.39, 95% CI; 1.020-1.887; p = 0.04). However, Apa1 SNP was not shown statistically significant association with obesity (OR = 1.05, 95% CI; 0.681-1.625, p = 0.82) across all models. On contrary, neither Fok1 nor Apa1 SNPs were significant predictors of family history of T2D across any models (p > 0.05). In Table 9, logistic regression analysis performed to determine allelic association with obesity and FH of T2D. An A allele when compared to C and G alleles, showed statistically insignificant association with obesity and FH of T2D regardless of the severity of vitamin D deficiency across any model unadjusted and adjusted. Therefore, A allele is not a predictor of obesity and FH of T2D across mild and severe vitamin D deficiency groups (p > 0.05). Similarly, logistic regression analysis of Fok1 and Apa1 models (additive vs. dominant) for obesity and FH of T2D across vitamin D deficiency categories (mild, severe) did not exhibit significant association (p> 0.05; Table 10). While the models showed potential trends, the statistical tests failed to reach significance at p <0.05. For Fok1, the dominant model in mild vitamin D deficiency showed an OR of 3.25 (95% CI: 0.28-38.33; p = 0.35) for the odds of developing FH of T2D. Similarly, in severe vitamin D deficiency, the dominant model for Fok1 SNP yielded an OR of 3.45(95% CI, 0.34, 35.1, p = 0.30) for obesity. Although statistical significance was not achieved, the Fok1 SNP dominant models demonstrated higher odds for developing obesity in severe vitamin D deficiency and FH of T2D in mild vitamin D deficiency.
Table 8: Binary logistic regression models to predict factors associated with obesity (Non-Obese vs. Obese) and FH of T2D.
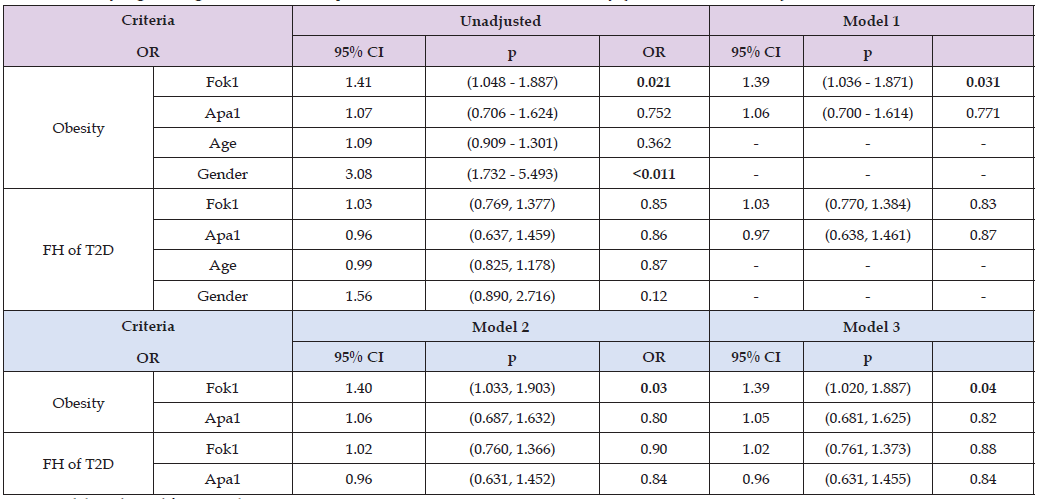
Note: Model 1: adjusted for age only.
Model 2: adjusted for gender only.
Model 3: adjusted for both gender and age.
‘-’ indicated empty cells Bold p-values showed statistical significance (p < 0.05)
Table 9: Logistic regression analysis of allelic association (A vs C and A vs G) with obesity and FH of T2D against vitamin D deficiency.
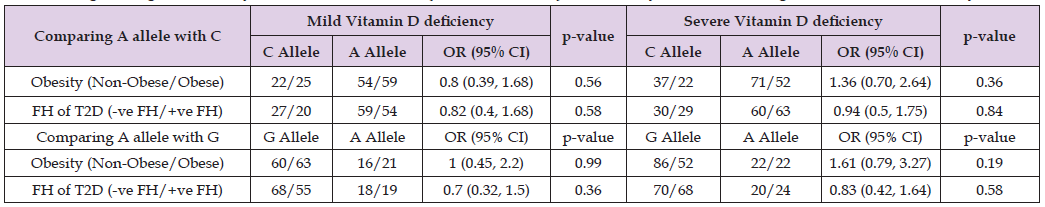
Note: Model adjusted for age and gender. Mild Vitamin D Deficiency = 25.1-50 (nmol/L), Severe Deficiency = <25 (nmol/L).
Table 10: Logistic regression analysis of Fok1 and Apa1 models (Additive versus Dominant) for obesity, FH of T2D across vitamin D deficiency.
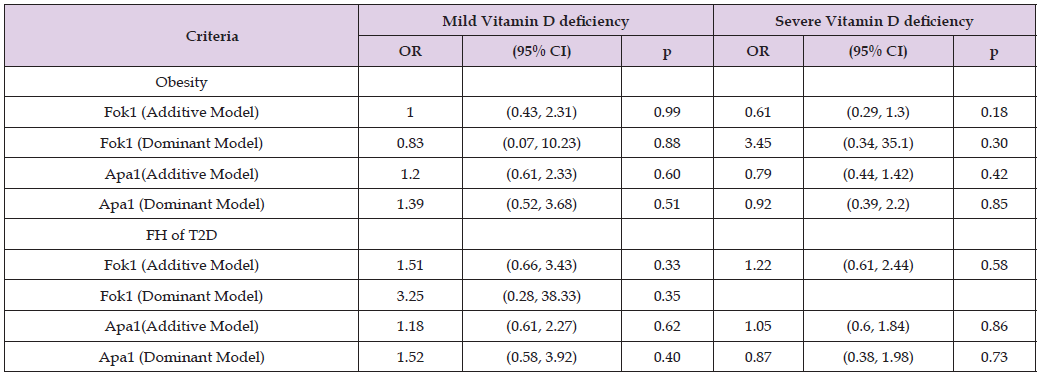
Note: Fok1 (Additive Model) = (AA vs. GA vs. GG), Fok1 (Dominant Model) = (AA vs. GA+GG),
Apa1 (Additive Model) = (AA vs. CA vs. CC), Apa1 (Dominant Model) = (AA vs. CA+ CC).
Obesity (dependant binary outcome) was defined as: Non-Obese = (BMI <25) and Obese = (BMI ≥25).
Mild Vitamin D Deficiency = 25.1-50 (nmol/L), Severe Vitamin D Deficiency ≤25 (nmol/L).
Models adjusted for age and gender. P-value <0.05 was considered statistically significant.
In Table 11, while determining the association of Fok1 and Apa1 SNPs with metabolic syndrome components, it was found that Fok1 and Apa1 SNPs were not significant predictors of metabolic syndrome (p > 0.05). However, vitamin D deficiency was significantly associated with some of metabolic syndrome components such as waist circumference (p < 0.001), BMI (p = 0.046), systolic blood pressure (p = 0.002), and triglycerides (p = 0.006), but fasting blood sugar (0.546), diastolic blood pressure (0.976), HDL (0.220) showed insignificant association. Therefore, vitamin D deficiency displayed greater impact on some of metabolic syndrome components. In gender specific logistic regression analysis, Fok1 and Apa1 SNPs were not significant predictors of obesity or FH of T2D across mild and severe vitamin D deficiency in both males and females (p > 0.05). While some patterns were observed, such as an OR of 3.33 (95% CI, 0.308-36.11, p = 0.320) for theFok1 additive model in females with mild deficiency, and an OR of 3.33 (95% CI, 0.285, 39.04, p = 0.340) for Apa1 dominant models in males with mild vitamin D deficiency, although statistically significant was not achieved. Therefore, no influence of Fok1 and Apa1 SNPs were observed on vitamin D deficiency, FH of T2D and gender specific risk of obesity (Table 12).
Table 11: Associations of Fok1, Apa1 models, and Vitamin D levels with the components of the Metabolic Syndrome.

Note: BMI = Body Mass Index, FBS = Fasting Blood Sugar, BP = Blood Pressure, TG = Triglycerides, HDL = High-Density Lipoproteins, Tchol = Total Cholesterol, LDL = Low-Density Lipoprotiens, ApoA1 = Apolipoprotein A1, ApoB = Apolipoprotein B. P-value was generated using non-parametric Kruskal-Wallis H test; except for HDL, parametric ANOVA test was used. P-value <0.05 was considered statistically significant. Shapiro-Wilk test was used to assess normality (not shown). Significant p-values were bold.
Table 12: Gender specific logistic regression analysis of VDR Fok1 and Apa1models for obesity and FH of T2D across Vitamin D deficiency.
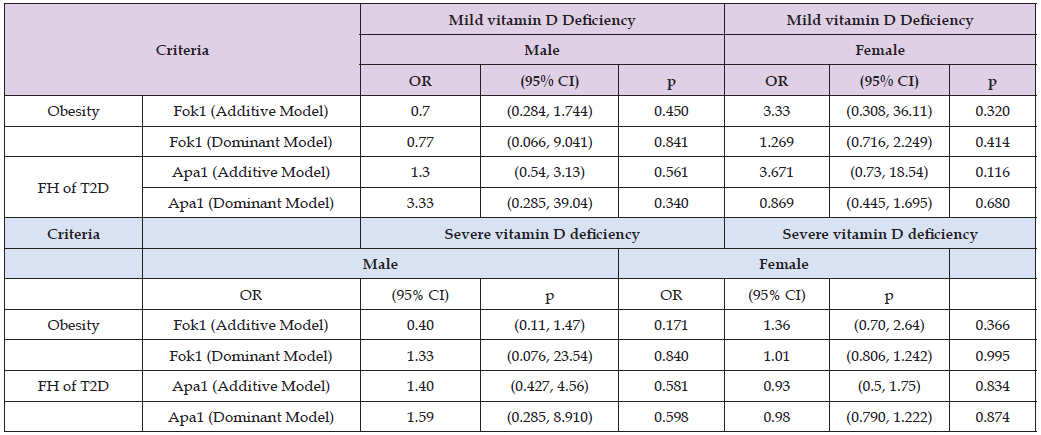
Note: Fok1(Additive Model) = (Fok1 (Dominant Model), Apa1 (Additive Model) =Apa1 (Dominant Model) Models adjusted for age.
In this cross-sectional study of 201 medical and dentistry students (99 males, 102 females) having mean age of 21.0 + 2.0 years and mean BMI of 25.35 +4.79 (Kg / m2), a statistically significant prevalence of obesity and vitamin D deficiency was observed. Males exhibiting higher frequency of obesity (59.6%), while females reported a higher frequency of severe vitamin D deficiency (67%). Additionally, more females (55.9%) had positive FH of T2D compared to 44.4% males, though difference was not significant (p = 0.105). Linear and logistic regression analysis were performed to determine the effects of Fok1, Apa1 SNPs, and vitamin D deficiency on the risk of developing obesity and T2D in young adults with FH of T2D.Fok1 and Apa1 SNPs were not identified as significant predictors of BMI (continuous and categorical), vitamin D levels, obesity, or FH of T2D across all models (p > 0.05). However, in the allelic and genotype association analysis, a significant difference was found in Fok1 genotype frequencies compared to global data (p = 0.028), although the allele frequencies consistent with global patterns (p = 0.077). These findings are contradictory to the literature as Chen et al carried out a meta-analysis published in 2019 that compared 1657 healthy controls with 1188 obese participants, showing no associations of Fok1 to increase the risk of obesity. Additionally, Chen et al also found that although Apa1 and Fok1 are not significant predictors, the wild type G-Allele of VDR Fok1 significantly increased the risk of obesity (Chen, et al. [23]). Furthermore, Central European Caucasians study found that VDR Fok1 and Apa1 was not associated with increased risk of obesity and T2D (Bienertová-Vašků, et al. [24]). However, literature review showed that VDR Apa1 and Fok1 were associated with impaired fasting glucose, T2D, and insulin resistance disorders in some certain populations, but no VDR Apa1 association found with positive FH of T2D. Thus, they were not risk factors for future development of T2D in healthy young adults (Han et al., 2017). Vitamin D deficiency was found to be significantly associated with certain components of metabolic syndrome, including waist circumference (p < 0.001), BMI (p = 0.046), systolic blood pressure (p = 0.002), and triglycerides (p = 0.006). Furthermore, SNPs genotype models exhibiting higher odd ratios trends.
For example, in the Fok1 dominant model, severe vitamin D deficiency showed higher odd ratios for obesity (OR = 3.45, 95% CI: 0.34- 35.1, p = 0.30), while mild vitamin D deficiency exhibited increased odd ratios for FH of T2D(OR = 3.25, 95% CI: 0.28–38.33, p = 0.35). Similarly, in the Apa1 dominant model, males with mild deficiency demonstrated higher odds for FH of T2D (OR = 3.33,95% CI: 0.308– 36.11, p = 0.32), although it failed to reach statistical significance. Both Fok1 and Apa1 did not show any significant association (p-value > 0.05) with any of the components of the Metabolic Syndrome, which is consistent with the findings of meta-analysis published in 2020 which also showed neither Fok1 nor Apa1 were associated with the metabolic syndrome (Totonchi, et al. [15]).
The similar findings were reported in a meta-analysis of 71 studies published in 2018 explained that vitamin D deficiency increased the risk of developing hyperglycemia both in diabetic and non-diabetic subjects (Rafiq Jeppesen, et al. [25]). These findings were supported by another meta-analysis of 28 studies published in 2017 which also concluded that a higher vitamin D status significantly lowered the risk for T2D (Ekmekcioglu, et al. [26]). Although Vitamin D deficiency contribute to cause many chronic diseases, it is still controversial in the current literature if it contributes to obesity (Vranić, et al. [27]). On the other hand, the literature shows that obesity leads to vitamin D deficiency, since it is a lipid soluble vitamin and can sequestrate through adiposity, leading to reduced levels when measured (Holmlund‐Suil, et al. [28]). This is consistent with our finding that individuals with optimal vitamin D levels had the lowest waist circumference (p-value = 0.001), BMI levels (p-value=0.046) respectively. The association between vitamin D deficiency and obesity is well documented; however, it is not shown that this association is bidirectional (Vranić, et al. [27]). The combined effect of vitamin D deficiency found to be associated to certain metabolic syndrome components (p < 0.05) which may increase risk of obesity and FH of T2D.
The regional population specific studies in surrounding countries of Kuwait also showed high prevalence of Vitamin D deficiency; 25% in preschool children (AbdelKader, et al. [29]), 84% in T1D children (Liu, et al. [30]), 81.21% in Adolescents (Al-Taiar, et al. [31]), 64% in male athletes (Alkoot, et al. [32]), and 36.3% in adults (Zhang, et al. [7]). However, no published studies specifically demonstrated the vitamin D status for healthy young adults in Kuwait. Furthermore, countries within the same geographic area demonstrated comparable observations, where a cross-sectional study published in 2019 for Qatar consisted of 102342 participants (adults between 18 and 65 years old) demonstrated 71.4% vitamin D deficiency (Zainel, et al. [33]). In addition, a study of 7924 Emirati patients published in 2016 showed vitamin D deficiency of 85.4% (Yammine, et al. [34]), whereas in Saudi Arabia 87.8% vitamin D deficiency was reported in healthy adult males aged 20-74 years (Ardawi, et al. [35]). Globally, the prevalence of vitamin D deficiency widely varied s 34% in Africa(Mogire, et al. [36]), 28%in the Brazil (Pereira-Santos, et al. [37]), 20% in Australia( Malacova, et al. [23]). Other studies showed that healthcare workers widely suffered of vitamin D deficiency, emphasizing the need to raise awareness among the future healthcare educators, providers and the public. A systemic review of 71 peer-reviewed articles, published in 2017, revealed that vitamin D deficiency is present in different occupations. The review reported 72% vitamin D deficiency among healthcare students, 65% medical residents, 46% practicing physicians, and 43% nurses, aligning somehow with the prevalence in this study (Sowah, et al. [38]). The global pandemic emphasized the importance of implementing awareness campaigns to encourage healthy young adults to increase their exposure to sunlight, the main source for vitamin D, to encourage the consumption of vitamin D fortified food or supplements (Pludowski, et al. [39]).
Limitations and Implications
A few of limitations and implications of this study are: At first, there may be insufficient sample size (n = 201; males = 99; females = 102), to determine existing genetic association [40,41]. We concluded that it would be biased to assess genetic vulnerability for obesity in our age-specific healthy young college medical and dentistry students, especially in a cross-sectional study without any follow-up studies. This age group is motivated to practice healthier lifestyle, as they are culturally primed for marriage, and are the future medical providers and healthy lifestyle advocates. These reasons would motivate our age-specific population to adapt more protective behavior that would partly counteract the genetic susceptibility for obesity; as a result, this lifestyle would provide protective factor to determine any genetic association if it exists. Our negative results for our age-specific and ethnicity-specific subpopulation in regard to obesity and T2D, are still useful as a reference for future studies and meta-analysis, representing healthy young adults in Kuwait.
There was no significant effect of Fok1 (rs2228570) or Apa1 (rs7975232) on obesity or T2D in healthy young adults of Kuwait, using both additive and dominant genetic models. The GG genotype of VDR Fok1 showed significant association with obesity while VDR Apa1 did not. None of the SNP genotype were significant predictors for impending risk of obesity and T2D. Due to the insufficient number of participants in the study group who were diagnosed with this metabolic syndrome, we studied the possible association of its components and no significant results were found for SNP genotypes but statistically significant association of vitamin D deficiency with certain metabolic syndrome components were identified. The study presented some limitations of unequal sample size distribution among male and female. The cross-sectional study design may limit to determine the long-term impact of genetic model on outcomes because no follow up was carried out. Future researchers are suggested to conduct longitudinal studies to capture long-term impact.
Ethics Approval
The study was approved (Approval Reg # 1351-2021) by the Joint Research Ethical Committee of the Ministry of Health of Kuwait and Kuwait University’s Faculty of Medicine. The committee evaluated and approved Arabic and English parental consent forms for minors under 21 years of age and adult consent forms.
Funding
This research was supported by the Kuwait University College of Graduate Studies and awarded as part of a Master’s Degree Thesis (0545598).
Consent to Participate
The consent papers clearly stated the project’s goals, risks, and benefits, the blood collection technique, and members’ rights to data confidentiality and to exit the study at any time. As requested by the Ethical Committee, the consent form stated that all blood samples were discarded after lab examination and would not be used for future study.
Consent for Publication
Not Applicable.
Availability of data and material
The article is part of thesis of master degree which is available at National Library of Kuwait (ISBN: 978-99906-1-546-3). The datasets generated and/or analyzed during the current study are available in the [National Libraryof Kuwait] repository, [https://nlk.portal.medad. com/en/search-results/c2VhcmNoX2FwaV9zb2xyXm5ld19zb2xyOjpmYTJjMGU0OS0yYzhmLTU3N2QtOTY4NS01MjRhZjdhYmQzNDA=]
Competing Interests
There was no financial or non-financial competing interests declared among authors.
Acknowledgements
I would like to thank the College of Graduate Studies of Kuwait University for funding the research. Mubarak Al-Kabeer Hospital (MKH) for processing our study blood samples for biochemistry and hematology blood tests.


