Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
García Viveros María Ricarda1*, Hernández Ojeda Humberto2, Silva Cañetas Carmen Sofía del Socorro3 and Torres Hernández Rosa María4
Received: March 12, 2025; Published: March 19, 2025
*Corresponding author: Torres Hernández Rosa María, Clinical research Faculty of Medicine, Universidad Veracruzana, Mexico
DOI: 10.26717/BJSTR.2025.61.009540
Introduction: Peripheral nervous system disorders caused by diabetes are well-researched, but changes in the
central nervous system remain less explored. Diabetic retinopathy (DR) is asymptomatic in its early stages, making
detection difficult. The P100 wave of the visual evoked potential (VEP) of reverse pattern has been useful in
assessing the post-retinal visual pathway and detecting changes before structural damage occurs.
Objective: To determine the relationship between visual pathway impairment and visual evoked potential of
reverse pattern in patients diagnosed with type 2 diabetes mellitus (DM) for five years.
Methodology: A cross-sectional analytical study was conducted on 21 patients diagnosed with type 2 DM for
five years. These patients underwent reverse pattern visual evoked potential studies, fundoscopy, and serum
HbA1c concentration measurements. Cross-tabulations were analyzed, and a p-value (>0.05) was obtained.
Results: The study included 21 patients with a mean age of 53.71±11.40 years, comprising 3 men and 18 women.
Among them, 3 patients with normal fundoscopy had a latency of >114 ms, corresponding to 14.3% of the
sample. Seventeen patients had normal fundoscopy and latencies between 89 and 114 ms, accounting for 81%.
Only one patient had retinopathy but exhibited a latency of 89 to 114 ms, representing 4.8%.
Conclusions: Reverse pattern VEP is a useful diagnostic tool that allows the association of P100 prolongation
with metabolic alterations in HbA1c levels and the progression of type 2 DM without retinopathy.
Keywords: P100 Association; Diabetic Retinopathy; Visual Pathway Impairment
Abbreviations: DM: Diabetes Mellitus; VEP: Visual Evoked Potential; DR: Diabetic Retinopathy; PKC: Protein Kinase C; AGEs: Advanced Glycation End Products; VEGF: Vascular Endothelial Growth Factor; PDR: Proliferative Diabetic Retinopathy; MAP: Mitogen-Activated Protein; VEP: Visual Evoked Potential; PLA2: Phospholipase A2; HIF-1: Hypoxia-Inducible Factor 1
Diabetes mellitus is a chronic disease characterized by heterogeneous metabolic disorders, leading to abnormally elevated fasting glucose levels (100–125 mg/dL or 5.6–6.9 mmol/L) in venous plasma. This condition results in several complications, including diabetic retinopathy (DR), which is a progressive disease caused by vascular damage characterized by increased permeability, capillary involvement, and retinal deterioration [1,2]. Various metabolic pathways contribute to hyperglycemia-induced vascular damage, including the polyol pathway, the accumulation of advanced glycation end products (AGEs), the protein kinase C (PKC) pathway, and the hexosamine pathway. The initial response of retinal blood vessels to hyperglycemia includes vasodilation and changes in blood flow, considered a metabolic autoregulation to enhance retinal metabolism in diabetic individuals. This process is associated with the formation of microaneurysms, the earliest clinical sign of DR. Retinal ischemia/hypoxia leads to the upregulation of vascular endothelial growth factor (VEGF) through hypoxia-inducible factor 1 (HIF-1) activation. Additional evidence suggests that phospholipase A2 (PLA2) elevation in diabetic conditions also triggers VEGF upregulation, which is crucial in proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME). VEGF is believed to increase vascular permeability by inducing the phosphorylation of tight junction proteins such as occludin and zonula occludens-1 (ZO-1).
Furthermore, as an angiogenic factor, VEGF promotes endothelial cell proliferation through mitogen-activated protein (MAP) activation. Other angiogenic factors, such as angiopoietins (Ang-1, Ang-2), are also involved [3-5]. This pathology is classified into two phases based on its progression and is considered the leading cause of preventable blindness in adults aged 20 to 74 years. The estimated national prevalence of blindness in Mexico ranges from 0.4% to 1.5%, and 2.4% to 7.0% of the population has visual impairment. [1,5-7] Several methods are available for diagnosing DR, including assessments of corrected visual acuity, intraocular pressure, biomicroscopy, gonioscopy, and fundoscopy under pharmacological mydriasis, including vitreous, posterior pole, and peripheral retina evaluation performed by an ophthalmologist. However, another underutilized diagnostic tool is the visual evoked potential (VEP), an electrophysiological study of the primary visual cortex in response to visual stimuli. VEP provides crucial diagnostic information about the functional integrity of the visual system from the retina to the occipital cortex and is sensitive to subclinical disorders of the optic nerve and macula. There are two primary stimulus protocols for recording VEP: reverse pattern (checkerboard) and flash, with the former being the most commonly used in adult patients [1,2,8-16]. The objective of this study is to analyze the relationship between visual pathway impairment and reverse pattern visual evoked potential in patients diagnosed with type 2 diabetes mellitus for five years.
A cross-sectional analytical study was conducted on patients diagnosed with type 2 diabetes mellitus who were beneficiaries of the ISSSTE healthcare system. Prior authorization from the Ethics and Research Committee of the Veracruz High Specialty Regional ISSSTE Hospital was obtained, along with informed consent from the patients. Inclusion criteria: patients with type 2 DM diagnosed for less than five years, aged >18 years, of any sex, ISSSTE beneficiaries, asymptomatic and/or with visual deficits, and evaluated by ophthalmology with fundoscopy. Exclusion criteria: advanced retinopathy, ocular tumors, glaucoma, and patients without an ophthalmology evaluation. Elimination criteria: multiple sclerosis and incomplete studies. Patients were referred by the ophthalmology department and the MIDE program to the physical rehabilitation service. The rehabilitation physician recorded the following parameters in the database: HbA1c, fundoscopy results, duration of diabetes diagnosis, presence of symptoms, and their description. Electrodes were placed on the scalp following the International 10/20 System using a one-channel setup with an active Oz electrode, referenced to Cz and Fz as ground. After preparing the area, electrodes were placed, and impedance was checked to be below 5 kOhms. A reverse pattern monitor was positioned 1.5 meters away, and the seated patient was instructed to fixate on a red point at the center of the checkerboard without deviating gaze, with permission to blink.
A conditoning test was conducted, as some patients may experience dizziness due to alternating black-and-white patterns. If tolerated, the definitive test was performed. The test was conducted on both eyes separately, with contralateral occlusion. P100 latency and amplitude were measured, and the results were recorded in the patient database. Statistical analysis of qualitative variables was performed using frequency and percentage, while quantitative variables were analyzed using mean and standard deviation. Group differences were assessed using one-way ANOVA and post hoc tests for homogeneity of variances. Associations between qualitative variables were analyzed using cross-tabulations and Fisher’s exact test (p > 0.05).
A total of 21 patients with type 2 diabetes mellitus (DM) were studied. The mean age was 53.71±11.40 years: 49.20±6.2 years for the diabetic group, 57.27±9.6 years for the prediabetic group, and 50.40±17.5 years for the healthy patients (p=.337). Regarding the time since diagnosis, the mean for diabetics was 3.20±1.4 years, for prediabetics 2.73±1.7 years, and for the normal group 3.60±1.6 years (p=.617). The distribution by sex was as follows: among females, 14.2% had diabetes, 61.9% were prediabetic, and 9.5% had normal glucose levels; among males, 9.5% had diabetes, and 4.7% were prediabetic (p=.168). (Table 1) The P100 latency in patients with type 2 DM was 116.46±20.43 ms, in prediabetics 105.77±8.04 ms, and in patients with normal HbA1c serum levels 107.50±5.44 ms. One-way ANOVA and Post hoc tests resulted in F (2.056) (p>0.05). When analyzing the P100 amplitude, the values were 7.45±3.94 for diabetic patients, 8.22±3.27 for prediabetics, and 9.22±3.23 for patients with normal HbA1c serum levels. One-way ANOVA and Post hoc tests resulted in F (.718), F (p>0.05) (Table 2).
Table 1: Characteristics of patients with type 2 DM in visual pathway impairment and visual evoked potential of reverse pattern.
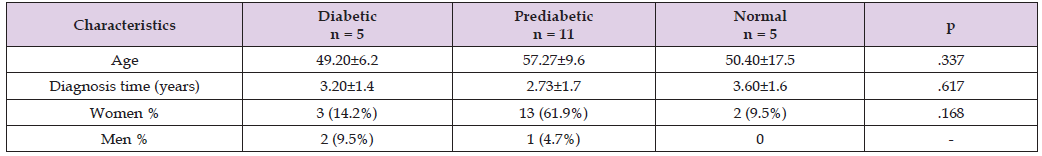
Table 2: P100 latency and amplitude in patients with type 2 DM, prediabetic individuals, and those with normal serum HbA1c values.

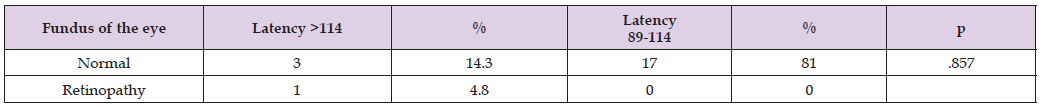
The P100 amplitude among the study groups showed a higher amplitude in patients with normal HbA1c serum values, but the statistical analysis using the Post hoc test indicated that the groups were homogeneous, with a significance value of .645. (Figure 1) Among the 21 type 2 DM patients included in the study, all underwent fundoscopy and pattern-reversal visual evoked potentials (VEP) testing. It was observed that three patients with a normal fundoscopy had a latency >114 ms (14.3% of the sample), 17 patients had normal fundoscopy and latencies between 89 and 114 ms (81%), and only one patient had retinopathy but with a latency between 89 and 114 ms (4.8%). Cross-tabulations were analyzed using Fisher’s exact test, p (>0.05) (Table 3) [17-21].
Table 3: Relationship between fundoscopic findings and P100 latency in patients with type 2 DM, prediabetic individuals, and those with normal serum HbA1c values.
Diabetic retinopathy (DR) is a specific microvascular complication of DM that affects 1 in 3 individuals with the disease. DR is the leading cause of vision loss in the economically active population. It has been reported that patients with severe DR experience poorer quality of life and a reduction in physical, emotional, and social well-being. There are several procedures to evaluate the visual pathway in these patients. The 2017 Clinical Guide for the Management of Diabetic Ocular Pathology by the International Council of Ophthalmology specifies that the initial examination of a patient with diabetes mellitus should include corrected visual acuity, intraocular pressure measurement, biomicroscopy, gonioscopy (if indicated), and fundoscopy under pharmacological mydriasis, including vitreous, posterior pole, and peripheral retina assessment. This should be performed using indirect ophthalmoscopy and/or slit-lamp biomicroscopy by an ophthalmologist. However, visual evoked potentials (VEP) are not considered at any stage of this assessment. Visual evoked potentials (VEP) are electrophysiological responses from the primary visual cortex to visual stimuli, which can be amplified and averaged from electroencephalographic signals. VEP provides crucial diagnostic information regarding the functional integrity of the visual system from the retina to the occipital cortex and is sensitive to subclinical disorders of the optic nerve and macula. This diagnostic tool has two main stimulation protocols for recording VEP: pattern-reversal VEP and flash VEP. The waveform components of pattern-reversal VEP include the negative N75 wave, the large positive P100 wave, and the negative N135 wave. Clinically, VEP is used in patients with optic neuritis and other demyelinating diseases.
VEP is useful for detecting hidden optic nerve damage before the onset of visual dysfunction. Alterations in nerve conduction can be quantitatively measured by changes in latency. The average latency value was higher in the type 2 DM group. When analysing the association between fundoscopy findings and P100 latency, three patients had normal fundoscopy but prolonged latency, although with no statistically significant value. However, based on the latency behaviour in response to abnormal HbA1c levels and the absence of structural damage to the optic pathway, we can consider that VEP may function as a predictor of visual impairment in patients with type 2 DM. This aligns with the study by Tuğba K et al., which highlights the prolongation of P100 latency in the glucose intolerance group compared to the control group. This finding is interesting as it suggests that this visual pathway assessment tool can predict damage before structural changes occur in the optic nerve or retina. The prolongation of P100 latency associated with abnormal HbA1c values is consistent with the study by Ozgur B et al., which emphasizes the significant difference between patients with HbA1c levels above 7% compared to those with lower or normal values. In our study, we also found patients with prolonged latency in the type 2 DM group with HbA1c levels above 6.5%, though without significant differences.
Nonetheless, these findings suggest that metabolic alterations, such as high glucose and HbA1c levels, disrupt visual pathway function, which is reflected in P100 latency prolongation. This can be explained by the fact that diabetic patients, at the time of diagnosis, may have already had the disease for several years, meaning that visual pathway damage is progressive and asymptomatic. Therefore, our findings support the potential usefulness of this diagnostic tool. Regarding P100 amplitude, in healthy patients, it was found to be above 9 mV, while in the other groups, it was attenuated but remained within normal and statistically non-significant values. It is important to note that this behaviour observed in our study is similar to the findings of Raghda S et al., who reported that P100 latency is prolonged and amplitude is attenuated in the DM and retinopathy group. This suggests that greater damage leads to slower optic nerve conduction, which is critical as it ultimately results in irreversible blindness. VEP may help in its early and timely detection.
Reverse pattern VEP is a valuable diagnostic tool for evaluating the visual pathway in patients with type 2 DM without retinopathy. It may serve as a predictor of structural optic nerve damage by associating P100 prolongation with metabolic alterations in HbA1c levels and the duration of DM progression. Moreover, it is a non-invasive and reproducible study.
Recommendations for improvement include optimizing processes by offering more appointment slots for patients with type 2 DM without retinopathy in the ophthalmology service and encouraging preventive consultations. Additionally, expanding the sample size and including a control group would enhance the study’s robustness.
• The authors declare that there is no conflict of interest.
• The article has not been published in any journal.


