Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
C Weir1, D Payne2, L de Bruin3, S Bashir4, J Reidl3 and L Whitby5*
Received: February 18, 2025; Published: March 04, 2025
*Corresponding author: L. Whitby, UK NEQAS for Leucocyte Immunophenotyping, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
DOI: 10.26717/BJSTR.2025.60.009513
Background: B-cell chronic lymphocytic leukemia (B-CLL) is the most common adult leukemia, requiring accurate
diagnosis and staging for effective treatment. Flow cytometry is a crucial diagnostic tool, with immunophenotyping
confirming B-CLL by detecting specific markers (CD5, CD19, CD23, and dim CD20). This study
evaluates the Sysmex XF-1600, a novel 10-color flow cytometry platform, in comparison to existing systems
(Beckman Coulter Navios and BD FACSLyric) across two international laboratories.
Methods: Thirty-three B-CLL samples from two sites (Royal Hallamshire Hospital, UK; Albert Schweitzer Hospital,
Netherlands) were analyzed using local methods and then analyzed using harmonized Sysmex XF-1600
protocols, which included standardized antibody panels and instrument settings cloned between laboratories.
Core antigen detection rates and median fluorescence intensity (MFI) values were assessed, comparing results
within and between laboratories.
Results: The XF-1600 demonstrated consistent antigen detection across both sites, identifying all core B-CLL
markers in all cases. In contrast, in-house methods occasionally failed to detect core antigens. MFI values varied
significantly within laboratories using in-house techniques but showed no significant inter-laboratory differences
using the XF-1600 (p<0.05). The XF-1600 approach minimized variability and reduced reagent use, optimizing
workflow efficiency.
Conclusion: The Sysmex XF-1600 provides a standardized, reproducible platform for B-CLL diagnosis, reducing
inter-laboratory variability and enhancing diagnostic accuracy. Its potential extends beyond B-CLL, offering a
solution for flow cytometry standardization in hematological malignancies.
Keywords: B-Cell Chronic Lymphocytic Leukemia (B-CLL); Flow Cytometry; Immunophenotyping; Standardization
Abbreviations: B-CLL: B-cell Chronic Lymphocytic Leukemia; WHO: World Health Organization; IWCLL: International Workshop on Chronic Lymphocytic Leukemia; NCCN: National Comprehensive Cancer Network; ASZ: Albert Schweitzer Hospital; PBS: Phosphate-Buffered Saline
B-cell chronic lymphocytic leukemia (B-CLL) is a slow-growing lymphoid neoplasm and the most common type of adult leukemia [1,2]. Clinical presentation varies widely, ranging from asymptomatic cases, diagnosed based on findings such as lymphocytosis, lymphadenopathy, or splenomegaly, to symptomatic cases with features including cytopenia, recurrent infections, and weight loss. Following diagnosis staging systems such as the Binet system (commonly used in the UK) and the Rai system (used in the US) provide valuable prognostic information and guide treatment decisions. The prognosis for B-CLL patients depends on a combination of clinical factors, including hematological parameters, cellular immunophenotyping, cytogenetics, and molecular analysis [3,4]. According to the most current guidance from the British Society for Hematology, [5] accurate diagnosis and staging are essential to inform management strategies, particularly with the advent of targeted therapies. These include B-cell lymphoma 2 (BCL- 2) inhibitors, such as venetoclax, and Bruton tyrosine kinase (BTK) inhibitors, for example, zanubrutinib and acalabrutinib [6], which have revolutionized the treatment landscape for B-CLL.
B-cell chronic lymphocytic leukaemia (B-CLL) is often diagnosed incidentally during routine blood tests, given its frequently asymptomatic presentation [7]. The diagnostic process typically begins with a full blood count, which often reveals lymphocytosis and may trigger further investigation. Subsequent morphological examination of a blood film demonstrates typical characteristic features associated with B-CLL: small lymphocytes with a high nuclear-to-cytoplasm ratio and a distinctive nuclear pattern showing clumped chromatin. To confirm the diagnosis, flow cytometry is performed, identifying the presence of a B-CLL population through specific immunophenotypic markers [8,9]. The diagnostic criteria for B-cell chronic lymphocytic leukaemia (B-CLL) established by the World Health Organization (WHO), International Workshop on Chronic Lymphocytic Leukaemia (IWCLL), and the National Comprehensive Cancer Network (NCCN) rely on the morphology and immunophenotype of neoplastic B-cells. These cells are characterized by the co-expression of CD19, CD5, and CD23, along with dim expression of CD20 and monoclonal surface immunoglobulin (sIg) expression [9-12] immunophenotype analysis of B-CLL typically reveals kappa or lambda immunoglobulin light chain restriction and the abnormal population’s positive expression of CD5, CD19, CD23, and dim CD20. Efforts to standardize the diagnostic markers have identified CD19, CD5, CD20, CD23, kappa, and lambda as “required” markers, while additional “recommended” markers such as CD43, CD79b, CD81, CD200, CD10, and ROR1 are valuable for differential diagnosis [9].
To streamline diagnostic workflows, several flow cytometry reagent suppliers now provide pre-cocktailed or dry reagent tubes designed to support laboratories in achieving consistent and accurate results. Recently, Sysmex launched the XF-1600 flow cytometer, a 10-colour system with 3 lasers, combined with VenturiOne software for data analysis from Applied Cytometry, UK. The XF-1600 allows users to ‘clone’ instrument settings between different instruments, to promote inter and intra-laboratory harmonization. An international collaborative study was initiated to compare the performance of a B-CLL screening panel on two harmonized Sysmex XF-1600 systems against current local platforms and testing strategies (Beckman Coulter Navios and Becton Dickinson FACS Lyric). The study assessed the suitability of all recommended antigens [9] (CD19, CD5, CD20, CD3 and CD23) within the Sysmex panel and compared them to local platforms. Kappa and lambda light chains comparison were excluded from this part of the analysis as harmonizing cell washing procedures at both sites was not feasible for this initial study and variances in washing could have affected the ability to compare results between the two sites.
Two evaluation sites were part of the collaboration, the Haemato- Oncology Diagnostic Service (HODS) at the Royal Hallamshire Hospital (RHH) in Sheffield, UK and Result Laboratorium (RL) at the Albert Schweitzer Hospital (ASZ), Netherlands. The samples used in this study were from patients already diagnosed with B-CLL. Following the completion of routine testing on each site, excess material (peripheral blood) was anonymized and then used for the collaboration study. As per local procedures, consent was not required as the samples were considered clinical waste and were not being subjected to any additional testing other than repeating that already undertaken. Cases of B-CLL were selected for this study as they are easily available, the immunophenotype is well known and is highly preserved between different patients. All samples consisted of B-CLL peripheral blood (n=33 (16 cases at ASZ and 17 cases at RHH) collected into tri-potassium EDTA (K3-EDTA) tubes, with all testing completed within 48 hours of sample draw. The in-house protocols for ASZ and RHH, together with the harmonized protocol are outlined below. For all the protocols, samples were adjusted to a leucocyte count of <20 x 109/L before preparation, and a minimum of 20000 events were acquired for each sample, regardless of other protocol differences. Data analysis software was used to class each antigen as positive/negative in each case and to measure the MFI of the core antigens in the gated population of interest.
Panel Validation and Harmonized Staining Protocol
As part of panel production, and to ensure that the recommended reagents were valid for use in the study for assessment of B-CLL, five normal patients were tested (using the following protocol and the harmonized panel B-CLL shown in Table 1) before commencing the study. For each sample, the minimum relative fluorescence intensity of positive and negative control populations was assessed in line with previously published recommendations [9]. For surface staining 100 uL of peripheral blood was added to a labelled 12 x 75mm polystyrene tube containing titrated antibodies as shown in Table 1. The tube was then vortex-mixed and incubated at ambient temperature for 20 minutes in the dark. Following this 2 mL of prepared Sysmex CyLyse FX was added to the sample. This was then vortexed and incubated at ambient temperature for 10 minutes in the dark. Samples were washed twice in an automated cell washer and the cell pellet resuspended in 0.5 mL of PBS. The sample was then vortex-mixed and acquired on an XF-1600 flow cytometer software version 5.0 and then analyzed using VenturiOne software V7.5. Example plots from a normal sample and a known B-CLL sample used to assess the suitability of the combinations/reagents can be seen in Figure 1.
Table 1: Antibody combinations and manufacturers used locally at ASZ and RHH. Recommended B-CLL Antibodies are shown in bold.
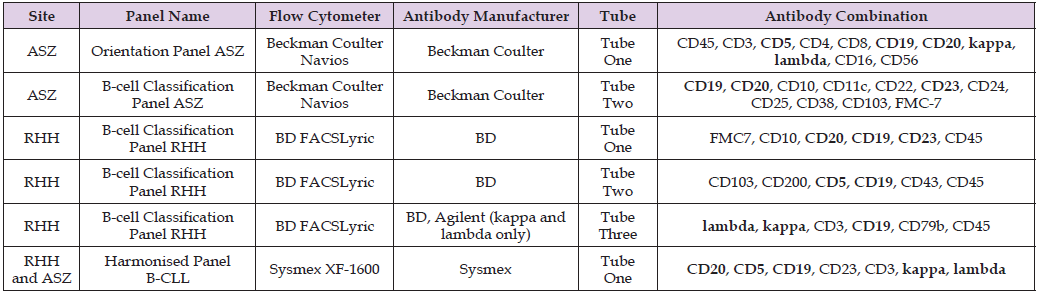
ASZ In-House Protocol
Before staining with monoclonal antibodies each sample was prepared as follows. Briefly, 1 mL of blood was lysed with ammonium chloride solution, followed by centrifugation and washing steps with phosphate-buffered saline (PBS) containing bovine serum albumin (BSA). The cells were then re-suspended in PBS with 1% BSA. For surface antigen staining, 50 μL of washed patient sample was added to each of two labelled 12 x 75mm polystyrene tubes containing the ASZ site panel pre-titrated antibodies as shown in Table 1. The tubes were vortexed for 5 seconds and incubated in the dark at ambient temperature for 20 minutes. Cells were then washed and re-suspended in 300μL of PBS with 0.2% BSA before acquisition on the flow cytometer. All samples were acquired per tube on a BC Navios flow cytometer, with data analysis being performed using Kaluza (version 1.4) and reanalyzed for the study using VenturiOne software V7.5.
RHH In-House Protocol
50 μL of patient sample was added to each of two labelled 12 x 75mm polystyrene tubes containing the RHH site panel pre-titrated antibodies, see Table 1. Washed cells were prepared for surface immunoglobulin (Kappa and Lambda) analysis by washing samples in warm PBS (37 oC), with the washed cells re-suspended in approximately 300 μL of PBS for use in testing with RHH Tube 3, see Table 1. The tubes were vortexed for 5 seconds and incubated for 15 minutes at ambient temperature in the dark. Following this, 1mL of prepared BD FACS lyse was then added. The tubes were vortexed again and incubated for a further 10 minutes at ambient temperature in the dark. Cells were then washed and re-suspended in 300μL of PBS before acquisition on a BD FACS Lyric flow cytometer. Data was analyzed using FACSuite software, version 1.5 and reanalyzed using VenturiOne software V7.5.
Instrument Harmonization Protocol
Cloning of the XF-1600 Panels and Settings Across Laboratories: The B-CLL panel and settings were created on the reference XF- 1600 instrument at RHH. This included a standard setup of a panel encompassing gain settings, titration of antibodies, compensation, dot plots and gating strategy [9]. The cloning procedure was then applied to the reference instrument. A medium fluorescent rainbow bead (CyFlow GainSet, Sysmex, Kobe, Japan) was acquired on the XF- 1600 using the B-CLL panel and all settings. The beads were then gated in an FSC v SSC log scale dot plot and the gate was applied to histograms for each fluorescent channel. A tight region was then placed to cover the peak observed in each channel and saved. The B-CLL panel and settings, along with the cloning information were sent to AZH for upload onto their device. Using the same lot number of CyFlow Gain- Set, AZH altered the gains in the panel to place the peaks into the gate in each channel of the template. This information was saved and applied to the B-CLL panel and intra-laboratory cloning was complete. The B-CLL samples identified at each site were then tested locally on the laboratory’s harmonised XF-1600 flow cytometer using the harmonised staining protocol described earlier to detect the presence/ absence of cell surface antigens. Each sample was acquired using the XF-1600 acquisition software, version 5.0. The data was then analysed using VenturiOne software V7.5.
Panel Validation
Assessment of five normal samples demonstrated that the required antibodies all exceeded the relative fluorescent intensities necessary for the detection of B-CLL [9], see Table 2.
B-CLL Samples
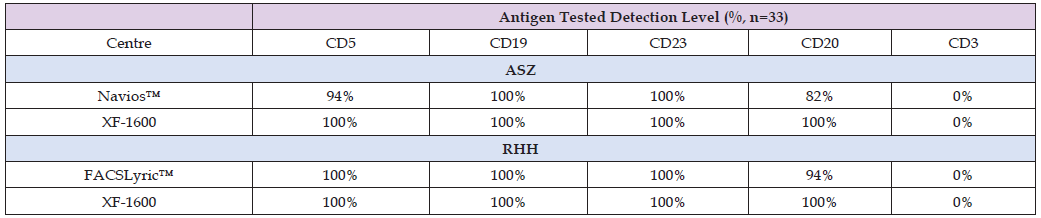
Following analysis of samples at each site, results were reviewed to determine the number of cases of B-CLL that expressed core antigens by each method (Table 3). Analysis of positive/negative for both in-house techniques and the standardized XF-1600 system demonstrated discrepancies between identical samples. In some cases, core antigens [9] were classed as absent using local methods. Whereas all core B-CLL immunophenotypic antigens (CD5, CD19, CD23, and CD20) were detected by the XF-1600 in all cases.
Table 3: Surface antigen staining comparison for 16 cases of B-CLL at ASZ and 17 cases of B-CLL at RHH showing antigen expression detection in the malignant population.
Assessment of Immunophenotype
Assessment of Staining: To assess the comparability of results between the systems (FACSLyric, Navios, 2 x XF-1600) and the two sites (RHH and ASZ), median fluorescence intensity (MFI) values for each antibody were compared using paired (intra-laboratory comparability) and unpaired (inter-laboratory comparability) t-test to identify any areas of significant difference (p <0.05). All antigens tested at RHH and ASZ showed a significant difference in the MFI values between the established local system and the XF-1600 using paired t-tests. (Table 4) Comparison of core antigen MFI values from the two in-house methods performed at ASZ and RHH showed a significant difference (p<0.05) by unpaired t-test between the two sites (Table 5).
However, a comparison of MFI values of core antigens using the harmonised protocol and the XF-1600 showed no significant difference (p<0.05) between sites for positively staining antigens. The negatively staining antigen CD3 did show a significant difference in overall MFI values.
This pilot study demonstrates the potential of the Sysmex XF- 1600 as a platform capable of being used in the harmonization of clinical flow cytometry applications, as illustrated here for the diagnosis of B-CLL across laboratories. The XF-1600 system, employing a single tube of standardized Sysmex CyFlow antibodies, met the recommended reagent specifications for B-CLL[9] and allowed the detection of B-CLL using fewer antibodies than the established in-house panels. The cloned flow cytometer protocol, combined with a standardised testing approach matched or outperformed traditional methods in detecting core diagnostic antigens (CD5, CD19, CD23, and CD20) in all cases tested. Of note, the standardized approach eliminated the interlaboratory variability observed with traditional methods, as shown by the consistent median fluorescence intensity (MFI) values across both evaluation sites. This highlights the reproducibility of the XF-1600 for multi-centre diagnostic applications. The observed differences in MFI values between in-house methods and the XF-1600 highlighted the limitations of traditional protocols, which often rely on local staff experience and knowledge, local instrument settings, panel design and antibody selections. These variations could lead to differences in diagnostic classifications because whilst current classification systems [13,14] use the same criteria for B-CLL based on the detection of essential antigens (CD19, CD20, CD5, CD23) both systems do rely on the strength of antigen expression for some disease states. As such any variation between testing sites, be it due to reagents, instrument setup or fundamental instrument design could be a potential source of diagnostic discrepancies and as such constitute a risk to patients. By providing reproducible results across laboratories, the Sysmex XF-1600 addresses this critical challenge in flow cytometry diagnostics i.e. the lack of standardization. A limitation of the study was the omission of the assessment of kappa and lambda on the harmonised protocol.
This was due to the inability to harmonise cell washing procedures at both sites and this would have impacted the comparison of results between the two sites. However, analysis of the full panel on normal and B-CLL samples at a single site (RHH, Figure 1) showed that the antibody combination is suitable for use and meets the requirements outlined by other groups[9] for the detection of B-CLL. Given the success of the harmonisation proof of concept for cell surface antigens, it follows that similar harmonisation is achievable for surface immunoglobulin staining, and this will be assessed in future studies of other lymphoproliferative disorders. Although this study only focused on B-CLL (a disorder with a relatively conserved immunophenotype) this principle can be extended beyond this specific diagnosis and could improve inter and intra-laboratory standardisation and best practice in flow cytometry. The Sysmex XF-1600 offers a scalable, modular and adaptable platform that could be applied to other haematologic malignancies with appropriate validation. However, additional studies encompassing a broader range of disorders and larger sample sizes are essential to confirm this.
The accurate diagnosis of leukaemia and lymphoma is essential for the implementation of therapies and treatments. The introduction of any new testing methodology must be fully validated to ensure it is fit for purpose. This initial study into the use of the Sysmex CyFlow antibodies on the Sysmex XF-1600 flow cytometer focused on B-CLL, due to the conserved immunophenotype across cases and the ready availability of material across both sites. In this study, the Sysmex Cy- Flow antibodies and cloning instrument set-up protocol on the Sysmex XF-1600 flow cytometer matched or outperformed established laboratory techniques when testing B-CLL samples for the core diagnostic surface antigens of CD5, CD19, CD23, CD3 and CD20. When comparing methodologies based on the MFI of the antigens tested there were significant differences seen in intra-laboratory testing at both sites between the in-house methods and the Sysmex XF-1600. With regards to inter-laboratory comparisons of MFIs, the in-house methodologies also showed a significant difference, whereas the Sysmex XF-1600 results from both sites showed no significant difference (p<0.05). Although numerous guidelines and publications have been produced to promote the standardization and harmonization of leukaemia and lymphoma testing by flow cytometry the uptake of these has been variable. The Sysmex CyFlow antibodies and cloning technique used on the Sysmex XF-1600 system provide a standardized and harmonised approach by design, and this is demonstrated by the results produced in this study. The use of the standardized panel was also seen to produce time savings at both sites compared to the local methods. Whilst not formally quantified as part of this study the harmonised testing cycle was much quicker, likely due to fewer pipetting steps and fewer tubes in test set-up. This also preserved sample volume and reduced the time of data acquisition.
When combined with the streamlined panel design, using fewer antibodies and reagents, these changes should deliver cost savings to laboratories. Further work would be required to exactly quantify the cost savings produced by the introduction of harmonisation by this method, as this would vary by site based on the time and reagents used for local procedures versus the harmonised methods. In addition, future research is required to examine the application of this approach to a wider range of haemato-oncological disorders and to ensure a suitable range of antibody combinations to allow diagnosis of other disease states. However, initial findings suggest that wide-scale adoption of this approach has the potential to improve consistency in diagnostic flow cytometry, benefiting patients throughout their diagnosis and treatment and by supporting international research collaborations.


