Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gitte De Boeck1*#, Ildikó V Tóth2*, Julie Jacobs1, Liliana MG Pereira2, Rui Quinta2, Erwin Pannecoucke1, Bas vander Woning1, Maria Gonzalez Pajuelo2 and Hans de Haard1
Received: February 14, 2025; Published: March 19, 2025
*Corresponding author: Gitte De Boeck, argenx, Ghent, Belgium, Fairjourney Biologics, Porto, Portugal, argenx, Ghent, Belgium
DOI: 10.26717/BJSTR.2025.60.009476
Phage display is a widely used technique for the discovery of antibodies because of the high throughput identification of target binding proteins from large genetic libraries. However, reformatting the phage expressed binding fragment to a full-length antibody often impacts binding properties, necessitating post-selection engineering and screening in a cumbersome process with multiple cloning steps. Using a specialized dual vector system named pFJ011, antibody fragments can be expressed on the phage surface fused to human (hu) Fc genes and can also be expressed as a bivalent antibody Fc fusion in E. coli. Unfortunately, these systems lack post translational modifications such as glycosylation of the Fc region, which is important for Fcɣ receptor binding or complement activation, thereby complicating the assessment of their effector functions to those of the mature, mammalian cell expressed antibody. In addition, impurities in E. coli produced antibody preparations do not permit potency measurements in bioassays. To streamline the antibody selection pipeline, we developed a triple vector system named pFJ014, allowing expression in both prokaryotic and mammalian cells without any additional cloning steps. The value of the dual and triple vector to identify high affinity binders was explored using GARP, a potential target for cancer immunology that lacks trusted immunochemistry compatible tools. Using a Western blot-based phage display selection method we were able to identify VHH huFc clones very well suited for GARP immunohistochemistry. As such, the triple vector poses itself as an emerging tool for phage display library generation, allowing a fast antibody characterization workflow without intermediate cloning.
Keywords: Phage Display; VHH; Antibody Library; Bivalent Display; GARP
Abbreviations: Fab: Fragment Antigen Binding; ScFv: Single Chain Variable Domain; sdAbs: Single Domain Antibody Fragments; mBiP: Binding Immunoglobulin Protein; FFPE: Prepare Formalin Fixed Paraffin Embedded
Phage display has proven to be a valuable tool in the discovery and evolution of therapeutic antibodies [1,2]. By fusing antibody fragments to the pilus protein pIII of phage M13 via genetic techniques, a phage display library is created enabling to link the phenotype (i.e., antigen binding) of each antibody fragment directly to its genotype [3,4]. Whilst such libraries have shown to provide a rich source of antibody diversity covering up to 10 orders of magnitude, the format of phage display nonetheless allows for high throughput screening for the selection of high affinity binders. Different antibody formats have been applied in the construction of phage libraries including fragment antigen binding (Fab), single chain variable domain (scFv) and single domain antibody fragments (sdAbs) [5-8]. Being intrinsically part of an antibody, Fabs are the most “natural” of the recombinant antibody fragments and due to their more difficult expression in bacteria, are displayed in a monomeric fashion allowing the selection on true affinity [9]. scFvs, being small monomers, are expressed more efficiently in bacteria, leading to multimeric display on phage. However, scFv have a tendency to self-aggregate leading to higher order surface expression molecules and, consequently, an increased apparent target affinity due to avidity effects [9]. The variable domains (VHH) of the camelid heavy chain antibodies are attractive as they are small (around 15 kDa) and generally have a very high solubility and stability. sdAbs, when derived from immunized camelids, are strictly monomeric, but multimeric when displayed on phage because of their very efficient expression in E. coli [10].
The antibody fragments displayed on phage have to be reformatted into IgG by recloning in eukaryotic expression systems. Consequently, the avidity of monomeric Fab or VHH binding to a target can’t be compared with a bivalent IgG, leading to rather different binding and/or neutralizing potencies for the phage displayed antibody fragments and the mammalian cell expressed IgGs. Moreover, the Fc region of antibodies that might be produced in E. coli is not glycosylated, which is relevant for binding to Fcɣ receptors and activation of complement. In addition, bacterial impurities like endotoxins complicate testing in bioassays. Therefore, the initial hits from phage display selections must be converted into full length IgG for further biological testing, and this process, which requires additional cloning steps, is laborious and time consuming [11-13]. Several strategies have been developed to overcome these limitations, however there is no platform at present that combines 1) phage display selection of antibodies in the (therapeutic) format intended for their specific application, with 2) the expression of these antibodies in bacterial periplasm and mammalian cells.
In a search to facilitate phage display and bacterial and mammalian expression of bivalent IgG like antibodies while eliminating the need for in between subcloning steps, we devised a triple vector system for the expression of camelid heavy chain only, fused to human Fc (VHH huFc). In doing so, we took advantage of the small dimensions and flexibility in linker design of the camelid VHHs [13]. In a first step, the dual vector pFJ011, enabling both phage display and bacterial expression of bivalent VHH huFc, was created by introducing a modified human IgG1 hinge plus huFc in a phage display vector used for VHH display. In a second step, pFJ011 was adapted for expression in mammalian cells, resulting in the triple vector pFJ014. In this paper, proof of concept for both the dual and triple vector is provided based on screening of immune libraries for anti-GARP antibodies. GARP, also called LRRC32, is a transmembrane protein that is highly expressed on the surface of CD4+ regulatory T cells (Tregs) upon T cell receptor engagement. GARP associates with latent transforming growth factor beta (TGF-β), an important modulator for homeostasis and function of Tregs, and promotes its secretion and activation [14]. As such, GARP plays a role in enhancing the suppressive phenotype of Tregs and in maintaining Treg mediated peripheral tolerance. In cancer, GARP amplification supports cancer cell growth and dissemination [15]. Tissue expression profiling of GARP has been limited and only few groups investigated the potential role of GARP TGF-β in oncology. Accordingly, next to the design of a new phage display vector also a phage display selection protocol was validated to identify antibodies for immunohistochemistry based on a Western blot-based selection method on denatured GARP mimicking the state of the protein present in formalin fixed paraffin embedded cells [16,17].
Cell Line and Transient Transfections
Human embryonic kidney (HEK293T) cells (ATCC) cultured at 37°C, 5 % CO2 in DMEM supplemented with 10 % FBS and 2 μg/mL gentamicin were transiently transfected with the mammalian pCDNA3.1 expression vector, encoding full length human GARP (provided by Sophie Lucas, DeDuve Institute, UCL). The lipofectamine 2000 transfection protocol (Life Technologies) was used according to the manufacturer’s protocol. The construct was equipped with a hemagglutinin (HA) tag to enable sorting of GARP positive cells (FACS Canto), using a PE conjugated anti-HA tag antibody (Biolegend, Cat# 901533) and propidium iodide. For western blot, approximately 1 million cells were suspended in lysis buffer (4 % SDS, 7 % glycerol, 1.25 % β mercaptoethanol, 0.2 mg/mL bromophenol blue and 30 mM Tris HCl, pH6.8) and loaded on SDS PAGE as described below.
Vector Construction
The pFJ011 phage display dual vector was constructed based on the in-house standard phage display vector pAX001, which is being used for phage display of camelid heavy chain only (VHH) antibodies [18]. pAX001 allows the production of phage particles, expressing the individual VHHs as a fusion protein with a c-myc tag, a hexahistidine tag and the geneIII product. pAX001 was modified by introducing a BstEII/NotI cloning site for human IgG1 hinge Fc sequences, while retaining the cloning site for VHH sequences. In the human IgG1 hinge the first 5 amino acid residues were deleted. Successful cloning of the modified human IgG1 hinge plus Fc was confirmed by transformation of E. coli TOP10 using a standard heat shock protocol, and sequencing of one of the resulting colonies.
Next, pFJ014 was generated for expression in mammalian cells through modification of pFJ011. Cytomegalovirus (CMV) and the pA_ globin rabbit, obtained from the commercially available mammalian expression vector pD2610 v10 (ATUM), were introduced into pFJ011 according to the manufacturer’s instructions. Using pFJ014, VHH genes can be cloned in frame with murine binding immunoglobulin protein (mBiP) and human IgG1 Fc, via the restriction enzyme pair BstEII and BssHII [19].
Phage Library Generation
cDNA from llamas that delivered anti-GARP clones, as nicely described by Cuende et al., was selected for de novo generation of the VHH libraries [20]. Two different animals were used for library construction. Initial VHH PCR amplification was performed using non tagged forward and reverse primers annealing at VH leader and CH2 regions, followed by the introduction of restriction enzyme sites BstEII/BssHII (pFJ014) or BstEII/SfiI (pFJ011) via nested PCR amplification using tagged forward and reverse primers for annealing at the VHH FR1 region and the Hinge region. After restriction endonuclease digestion, VHH genes were ligated and transformed into the pFJ011 or pFJ014 phagemid vectors by using ECC TG1 cells (Lucigen). Library size (CFU) was measured by titration of E.coli, transformed with phagemid vector containing VHH genes, on LBagar/2 %Glu/ AMP plates. VHH insert percentage was measured via colony PCR using specific primers for pFJ011 or pFJ014 VHH genes.
Phage Display Selections
Phages from the anti-huGARP VHH libraries were enriched in two consecutive rounds of selections: panning selection in Round 1 and filter selection in Round 2. The first round of panning selection used boiled (95°C for 5 min) recombinant huGARP protein (R&D systems) as antigen, immobilized on MaxiSorp plates (NUNC). Human serum albumin (Sigma) was used as irrelevant protein control. Blocking was performed with 4% skimmed milk. Thereafter, 10 μl phage library was added to the plates, per selection condition, for 2h at RT. After washing, phages were eluted with trypsin. In the second round of filter selections, the recombinant huGARP protein sample was incubated for 5 min at 95°C under reducing conditions, 2 μg was loaded on SDS PAGE gel (Mini Protean TGX Gel 4 20% acrylamide) and blotted on a PVDF membrane in a Trans Blot Turbo Transfer system. The correct transfer of the proteins was verified by reversible red Ponceau S staining. Important, the PVDF membrane was activated with methanol for exactly 30 sec before transfer and re soaked in methanol 3 times, 10 sec each, after transfer. Bands containing target protein were cut from the dried membranes and blocked for 30 min RT with blocking buffer (2 % gelatin / 2 % skimmed milk / 20 % llama serum). 10 μl of output phages, selected in round 1, were pre incubated in blocking buffer prior a 2h RT incubation with the blocked membranes. After washing, membrane bound phages were eluted with 100 mM triethylamine (TEA), the pH of the TEA eluate was neutralized with addition of 1 M Tris-HCl buffer.
Screening by Western Blotting
Western blot analysis was based on methods described before [19-22] with modifications for detection of periplasmic extracts or purified VHH Fc fusions. Shortly, recombinant huGARP (R&D systems) or huSA (Sigma) were run on SDS PAGE gel under reducing conditions, blotted on PVDF membrane and blocked in 4% skimmed milk. Blocked membranes were incubated with purified polyclonal phage (1:1000 dilution), crude periplasmic extracts (1:50 dilution) from the different rounds of phage display selections, or with purified VHH huFc (2.5 μg/ml) or VHH moFc fusions (1μg/ml) for 1 h at RT. Phages were detected with anti-M13 (GE Healthcare, Cat# 27- 9421-01) 1:5000, periplasmic extracts and VHH huFc with goat anti- human IgG, Fcɣ Fragment Specific (JIR, Cat# 109-035-098) 1:5000 and VHH moFc with donkey anti-mouse IgG (H+L) (JIR, Cat#715-035- 150) 1:20000. All detection Abs were HRP conjugated diluted in 1 % skimmed milk and incubated for 1h at RT. Staining was established using the colorimetric HRP substrate TMB (Sigma). Finally, total cell lysates of GARP transfected versus parental HEK293Ecells (0.25 0.125 0.062 X106 cells) were loaded on gel, incubated with 1 μg/ml purified monoclonal VHH moFc and detected with HRP anti-mouse Ab as described above.
Screening by ELISA
Binding of selected clones as periplasmatic extract or full length production of the huFc fusions was validated by ELISA. Wells of maxisorp plates (NUNC) were coated with 1 μg/ml of recombinant huGARP (R&D systems) or huSA (Sigma) overnight at 4°C. After blocking in 4% skimmed milk, periplasmic extracts (1:5) or purified VHH huFcIgG1 (1:3 dilution series starting from 200 nM) were incubated for 1h at RT and detected with mouse anti-c-myc IgG (Roche, Cat#11667203001) 1:1000 dilution followed by an anti-mouse IgG HRP (JIR, Cat# 715- 035-150) 1:20000 dilution. TMB (eBioscience) was used for development and reaction stopped with H2SO4.
Expression levels of different CDR3 clones identified in both pFJ011 and pFJ014 were compared as periplasmic extracts by ELISA. Wells of maxisorp plates (NUNC) were coated with 5 μg/ml of goat anti-human IgG-Fc specific (JIR, Cat#109-005-098) overnight at 4°C. After blocking in 4% skimmed milk, periplasmic extracts (1:5) were incubated for 1h at RT and detected with anti-HIS-HRP (Miltenyi Biotec, Cat#130-092-783) 1:1000 dilution. TMB(eBioscience) was used for development and reaction stopped with H2SO4. A titration curve (3-fold serial dilution ranging from 0.6-0.0002 μg/ml) of a purified VHH-huFc antibody was used to determine the concentration of VHH huFc periplasmic extract (P.E.) in each samples.
Production and Purification of VHH Fc Triple Vector Constructs
Transfection and production of VHH Fc antibodies was assessed according to the standard protocol for mammalian expression in ExpiCHO S cells (Gibco). In order to be produced as mouse Fc fusions to be used in the immunohistochemistry experiments, variable heavy chains were subcloned into pcDNA3.1 mIgG1 Fc fusion expression vector prior to transfection. Nine days post-transfection, the antibodies were captured from clarified supernatants using MabSelect SuRe 5 mL columns (GE Healthcare) in ÄKTA pure 25. Acidic affinity purified protein fractions were neutralized by the addition of 1 M Tris HCl pH 8.0 solution. After buffer exchange to 1 x PBS pH 7.4 Hiprep 26/10 Desalting column (GE Healthcare), samples were concentrated in MWCO spin concentrators (Millipore Amicon Ultra 15) and the protein concentration was determined Nanodrop, (ThermoFischer) based on UV absorption and on the calculated molar extinction coefficient. One μg of purified sample was analysed by SDS PAGE under reducing and non reducing conditions according to standard protocols. The percentage of monomeric fraction was assessed by analytical size exclusion chromatography. Approximately 10 μg of sample was injected onto a Superdex 200 increase 5/150 GL equilibrated in 1x PBS, using an isocratic mode at a flow rate 0.45 mL/min for 10 min at 25°C. LPS levels of the final sample were quantified using the EndoZyme® II (Hyglos GmbH) following manufacturer’s instructions.
Affinity Measurements by SPR
The surface bound apparent affinities of the bivalent VHH moFc constructs were measured by surface plasmon resonance with a Biacore 3000 instrument. Recombinant huGARP (R&D systems) was immobilized (1000 resonance units) on a CM5 chip via amine coupling. During measurement, a flow rate of 30 μl/min was maintained and seven 2 fold dilutions (starting at 500 nM) in HBS EP buffer were tested for 500 s. After each dilution, the chip was regenerated by 2 injections of 10 μl glycine HCl (pH1.5). Kinetic constants were calculated using BiaEvaluation software.
Flow Cytometry
Binding of purified monoclonal VHH moFc fusions to HEK293T GARP and HEK293T WT cells was evaluated using an Attune™ NxT Flow cytometer and in a 3 fold dilution series (starting at 1.3 μM). APC conjugated goat anti-mouse IgG was used as a secondary antibody (BD Pharmigen, Cat#550826) at 1:250. All staining and washing steps were performed in PBS, supplemented with 2 mM EDTA and 0.5 % bovine serum albumin.
Immunohistochemistry
Finally, the use of VHH moFc for the detection of GARP on formalin fixed paraffin embedded biopsies was assessed by IHC. HEK293T GARP (positive control) and HEK293T WT cells (negative control) were pelleted, fixated in 4 % formaldehyde and stored in 70 % ethanol prior to dehydration and paraffin embedding. In addition, FFPE biopsies of tonsil, derived from the tissue bank of CellCarta with appropriate ethical approval and informed consent for research use, were used to determine specific binding to regulatory T cells in the interfollicular regions. To assess aspecific staining, a commercial mouse IgG1 isotype control (Abcam, Cat#18448) was included as a negative control. Four 4 μm thick FFPE sections were prepared and subjected to a multi-step optimization procedure on Ventana BM Ultra (Roche) to identify the optimal protocol for GARP expression. The staining quality was evaluated by experienced pathologists for two criteria: signal intensity and signal-to-noise ratio. The selected IHC protocol can be found in Table 1.
In Situ Hybridisation
To validate our findings from IHC, GARP mRNA expression was determined using ISH with the RNAScope® 2.5 assay and probe Hs LRRC32 (Advanced Cell Diagnostics, Cat#457638) on a Leica Rx instrument (Leica Microsystems Inc.) according to manufacturer’s instructions. Briefly, tissue was pre-treated with heat (15 min at 88°C) and protease (15 min at 40°C) prior to hybridisation of the target probe. Pre-amplifier, amplifier and alkaline phosphatase labelled oligonucleotides were sequentially hybridised followed by chromogenic substrate to produce red punctate dots. Tissue was counter stained with Mayer’s Haematoxylin.
Quality control of the FFPE samples was evaluated by positive control probe PPIB (Advanced Cell Diagnostics, Cat#313908) and negative control probe dapB (Advanced Cell Diagnostics, Cat#312038) prior to testing with Hs LRRC32 probe. Samples passed quality control if the average number of punctate dots per cell throughout the entire sample were greater than five, using the PPIB probe, and maximal 1 dot per 10 cells, using the dapB probe.
Generation and Design of the pFJ011 and pFJ014 Vectors
The phage display dual vector was constructed by introducing sequences encoding a modified human IgG1 hinge Fc (human IgG1 hinge, CH2 and CH3 domains) fragment in the pAX001 phage display vector. By including a partial hinge, which lacks the N terminal cysteine residue, we aimed to improve conformational flexibility and simultaneously avoid erroneous disulfide mediated multimerization. Successful cloning of the modified human IgG1 hinge Fc was confirmed by sequencing. The resulting dual vector pFJ011 serves two purposes in one single vector. On the one hand, the vector is suited for phage display campaigns as it allows expression of VHH hFc molecules displayed on filamentous phage as a fusion protein with pilus protein pIII. To accomplish this, it also contains an Ori F1 allowing the production of single stranded DNA and packaging of phage particles.
On the other hand, as the pFJ011 is equipped with the bacterial Ori pUC and a bacterial secretion signal upstream of the VHH, this vector enables production of the antibody in the periplasm for biophysical characterization. Finally, the amber stop codon (TAG) prevents the expression of the phage M13 derived pilus gene III upon expressing in the periplasm of non-suppressor bacterial strains (Figure 1). To allow expression in mammalian cells, the pFJ011 vector was further engineered by introducing the CMV origin sequence, the mBiP signal peptide and a rabbit poly(A) sequence (Figure 1). This resulted in pFJ014, which permits expression of VHH hFc fusion proteins in mammalian cells without compromising its ability to express fusion proteins on filamentous phage and in the bacterial periplasm [19].
Generation of Garp Specific VHHs Against Linear Epitopes
GARP TGF-ß1 is expressed on the surface of tumor infiltrating Tregs, and is involved in the inhibition of cytotoxic CD8 T cell responses against the tumor [20]. Some tumor cells even express GARPTGF- ß1 themselves to evade from immune attack. Tissue expression profiling of GARP in tumor biopsies is therefore of interest but has been hampered by the lack of good monoclonal antibodies suitable for immunohistochemistry (IHC). The procedure to prepare formalin fixed paraffin embedded (FFPE), golden standard for tumor biopsies, denatures proteins thereby losing conformational epitopes, leaving the only linear epitopes. Antibodies have the advantage in IHC because of their specificity and their avidity due to bivalency. Our phage vectors engineered for the display of Fc-fused antibody fragments provides the most optimal combination of the efficient phage selection, and screening in bivalent Fc VHH format without cloning.
To test the feasibility of our engineered vectors to streamline the selection and subsequent host indifferent expression of VHH molecules, GARP TGF-ß1 was chosen as a target for validation. VHH libraries from recombinant GARP TGF-β1 immunized llamas were constructed in both pFJ011 and pFJ014. The resulting immune libraries comprised above 5×107 independent colonies whereof minimally 95 % contained full size VHH inserts. Phage display based on panning and filter selections was validated to identify antibodies recognizing linear epitopes for the identification of anti-GARP binders suitable for immunohistochemistry. In a first round of panning, phages were absorbed on immobilized recombinant human GARP protein (huGARP), resulting in an enrichment with almost no background in the negative HSA control. Interestingly, a 100 fold higher enrichment was seen with the pFJ011 vector compared to the pFJ014 vector (data not shown). Due to the lower enrichment after round I for pFJ014, an extra identical panning round was performed before switching to the filter selections. This extra panning round was successful and resulted in a 10 fold better enrichment (data not shown). In the second round, filter selections were assessed using blotted antigens as representatives of denatured proteins. Although rather low number of phages were eluted from the membranes, a 10-100 fold enrichment could be observed when compared to human serum albumin (HSA) for both vector systems. The specific enrichment observed during selection progress was further investigated by western blotting incubated with polyclonal phage and periplasmatic extracts.
Using recombinant huGARP and incubation with polyclonal phage outputs from pFJ011, a clear specific signal at the correct molecular weight was observed after one filter selection. Of note, one round of panning selection was insufficient to enrich for huGARP binders (Figure 2). These results were confirmed on both vector systems pFJ011 and pFJ014 using periplasmic extracts generated from bacteria infected with the different phage display selection outputs. No background binding was visible for the negative HSA control (Figure 2). To validate these results, VHHs from periplasmic fractions were evaluated for binding onto huGARP using ELISA. Approximately 45 % of the individual clones derived from the pFJ011 vector system were able to bind the recombinant protein. Hit rates for the pFJ014 vector system derived were much lower with only 25 % of binding clones (data not shown). Next, to compare P.E. expression in both vectors, an extra ELISA assay was run where clones from different CDR3s identified in both vectors, were captured by an anti-huFc and detected via His-tag. The dot plot of concentration values of P.E samples -interpolated from a purified VHH-huFc antibody curve -from the same CDR3 family show that the lower percentage of binders for pFJ014 is likely due to the lower level of expression at the periplasmic level (Figure 3).
Sequencing data revealed an overall high diversity within the different clones whereof six CDR3 families were found back in both vector systems. Thereof, CDR3 family representatives were selected for monoclonal antibody expression. GARP binding was confirmed by western blot analysis. Taken together, these data demonstrate that both pFJ011 and pFJ014 vectors can be used for phage display and enabled the identification of specific binders against huGARP. Moreover, the filter selection approach permits identification of clones that bind linear epitopes.
Mammalian Cell Expression and Validation of pFJ014 Derived clones
VHH clones derived from the pFJ014 vector could be produced as full-length Fc fusions after transient transfection into a ExpiCHO S cells, a robust mammalian expression system to produce antibodies [23]. All antibodies were purified via Protein A affinity chromatography with a yield range of 200-1000mg/L for all VHH-Fc fusions. Next, purity and integrity of the purified proteins was assessed by SDS PAGE and analytical size exclusion chromatography (SEC). On SDS PAGE, the selected clones showed a single band with an expected molecular mass of 82 kDa as expected. Analytical SEC demonstrated monomeric percentage of Fc fusions higher than 92 %. Binding of the clones to huGARP was confirmed by ELISA and western blot (Figure 4) to assess the capacity of VHH-humanFc constructs to bind properly folded and denatured recombinant human GARP, respectively. Taken together, the VHH Fc fusions in pFJ014 vector could be produced to high yields, purified using a straightforward classical strategy, without affecting the target binding characteristics.
Target Binding Characterization of anti-GARP VHH Mouse Fc Constructs
An accurate evaluation of human antibodies for indirect immunohistochemical methods on human tissue samples cannot be achieved because of endogenous immunoglobulins that are co-detected by the secondary antibody. Therefore, a panel of 5 representative huGARP binding clones (01E08, 01G04, 01H01, 02D07 and 02D05) were reformatted and produced in the mouse IgG1 Fc (moFc) backbone. The correct size and purity of the antibodies was demonstrated by SDS PAGE (intact IgG) and SEC (percent monomer peak), and the binding of the VHH moFc to denatured GARP was confirmed by western blot (data not shown). Furthermore, monoclonal VHH moFc showed binding on huGARP transfected cell lysates of HEK293T cells when transferred on to PVDF membranes and failed to show binding on mock-transfected control (Figure 5). To assess the cellular binding properties, binding to huGARP was analyzed by flow cytometry using huGARP transfected HEK293T cells and their mock-transfected counterpart. As demonstrated in Figure 4, only clones 01G04 and 01H01 showed specific binding to huGARP expressing HEK293T cells.
On the contrary, aspecific binding was observed with clone 01E08. The other antibodies (02D07 and 02D05) showed limited binding affinity to huGARP under these non-denaturing conditions. Finally, SPR was used for the kinetic characterization of the interaction between the bivalent clones and immobilized huGARP. In general, all clones excluding 02D05 demonstrated at least a single digit nanomolar apparent affinity for huGARP. Three clones featured an off rate with at least 2.1E+05 M-1s-1 but differ in their on-rate. Whereas 01G04 showed an almost 3fold faster off rate than 01E08 (1.85E-03 s-1 and 6.19E-04 s-1, respectively) they have a similar apparent affinity of approximately 2.5 nM; no accurate measurement could be determined for 02D05, indicating off-rate of at least 1E-07 s-1. Finally, clone 02D07 combined kinetic parameters that both differed from the other clones to establish an affinity of 5 nM (Table 2, Figure 6). Combining this kinetic characterization with the data from the western blot and flow cytometric experiments, clone 01G04 was selected for further IHC validation, and clone 02D07 was selected as a backup.
Table 2: Summary of kinetic parameters estimated based on the Langmuir 1:1 binding model for lead monoclonal VHH-moFc antibodies to immobilized huGARP by SPR.
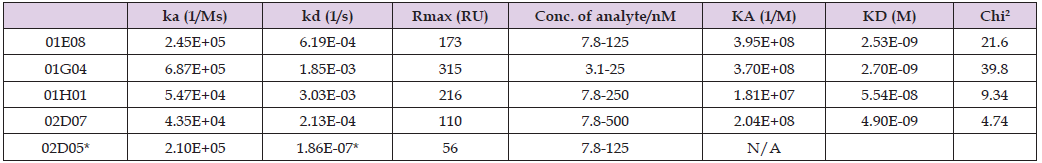
Note: *kd below the equipment limits (1.0E-06 1/s); N/A not applicable
Validation of anti-GARP VHH moFc Antibodies as IHC Reagents
In a final step, the VHH moFc antibodies 01G04 and 02D07 were evaluated for their suitability to detect GARP via immunohistochemical staining on formalin fixed paraffin embedded tissue. Optimization of the staining protocol was performed on GARP expressing HEK293T cells and the parental HEK293T cells. Additionally, tonsil was used to assess the specific expression of GARP on different cell types such as mesenchymal stromal cells, platelets, endothelial cells, Tregs and subsets of B cells. Parental HEK293T cells displayed no immunoreactive staining. On the contrary, clone 01G04 and 02D07 mediated strong staining patterns in the GARP expressing HEK293T cells while the staining of isotype controls remained negative. Similar results were obtained on the sorted GARP positive cells. On tonsil, specific staining in blood vessel and immune cell subsets was seen for both clones while the isotype control failed to provide signal after staining (Figure 7). Finally, Foxp3, a transcription factor marker specific for regulatory T cells, could be co localized within a subset of these immune cells by using serial cuts, confirming that the GARP-positive signal was detected on regulatory T cells. In concordance with published data, occasional non-specific staining of plasma cells and mast cells could be seen with both clones as well as the isotype control. Those cell types are often false positive in immunostaining methods that use avidin [24]. Overall, both clones could be used to screen for GARP expression on FFPE biopsies with the best S/N ratio for clone 01G04.
RNAscope® as Orthogonal Method for Accuracy Verification of GARP IHC Staining
Finally, to confirm specific binding of 01G04 and 02D07 to GARP, RNA in situ hybridization (ISH, RNAscope®) using a unique huGARP probe was performed. Specificity of the GARP RNAscope® probe was demonstrated by a clear staining on huGARP transfected HEK293T cells and absence of staining on parental HEK293T cells (Figure 7). In healthy FFPE tonsil tissue, GARP mRNA transcripts were mainly detected on endothelial cells and in the interfollicular area where the Tregs reside. Again, serial slides demonstrated an overall good association between the RNAscope® signal and the IHC staining pattern with 01G04 and 02D07 (Figure 7).
A plethora of display technologies are available for the selection and enrichment of high affinity biomolecule binders. Of those, phage display is arguably the most established one, and its application has already led to the discovery and approval of numerous mAb therapeutics. One strategy was the development of a bivalent display system that projected two copies of the antibody fragment (F(ab)’2) on the M13 bacteriophage, thereby allowing the phage to mimic the binding behaviour of natural IgGs during selection [25]. Although this strategy indeed facilitated the identification of lower affinity binders, the absence of an Fc part still necessitated conversion to full-length IgG to assess functional validation. Mazor et al. was the first to display full length IgGs on filamentous phage particles but the employed vector did not enable expression in mammalian cells without additional cloning steps [26]. Few years later, a dual host phagemid vector was developed allowing Fab phage display and expression in mammalian cells without subcloning steps [19].
However, this system did not provide an Fc in phage display, and therefore avidity effects could not be taken into account when screening the resulting monomeric “antibody fragments” using this system. Xiao et al. designed the splice vector system allowing a rapid screening of scFv Fc using bacterial expression before transfer to mammalian cells. This system enabled the expression of bivalent scFv Fc fusion proteins in both bacteria and mammalian cells but only permitted phage display of scFv without human Fc. As such, screening outputs of phage display still warrant subcloning into the dual pSplice vector for further testing [27]. Recently, Zhang et al. reported the first full length antibody phage display system on M13 phage. Nonetheless, similar to the strategy of Mazor et al., IgGs in the pDong3 system are still expressed in E. coli and thereby lack glycosylation [28]. As a result, when glycosylation does occur during mammalian cell expression, the produced antibodies are effector function competent, which might be important for their therapeutic efficacy. Also, mammalian cell produced antibodies can be assessed for neutralizing potency in bioassays, whereas bacterial impurities interfere with these cell based assays. Taken together, albeit substantial efforts, so far, no technology enabled phage display selection using prokaryotic and lower eukaryotic systems on the one hand, and full-length antibody characterization on the other, without the need of intermediate cloning steps. In this study, two variants (pFJ011 and pFJ014) of a standard phage vector, used for phage display of Camelid heavy chain only antibodies, were engineered. In both vectors a hinge Fc sequence was introduced allowing the display of VHH Fc fusions on the surface of a filamentous phage. pFJ014 was further equipped with the CMV origin sequence, the mBiP signal peptide and a rabbit poly(A) sequence, thereby eliminating the need for additional cloning for expression in eukaryotic cells. As such, pFJ014 enables direct screening in a therapeutically relevant format, including the correct post translational modifications. These two new vectors were used for the generation of VHH Fc fused immune libraries aiming to find antibodies that are suitable for detection of huGARP in immunohistochemical applications.
HuGARP has shown to be a key player in the immune suppressive tumour microenvironment by its expression on Tregs and its critical role TGF β1 activation. In addition, GARP is known to be expressed on a variety of cancers cells, which has positioned this protein as a potential valuable therapeutic target in oncology. Finally, GARP can be used as a biomarker for prognosis and therapeutic follow up by IHC [29]. Indeed, by allowing visualisation of the distribution and localization of biomarkers in different parts of a biological tissue, IHC represents an important tool in oncology for diagnosis, identification of therapeutic targets and prediction of treatment response. Therefore, the lack of a validated IHC protocol to identify GARP in cancerous tissue is strongly impeding the exploration of its role in oncology. Unfortunately, generation of anti GARP antibodies suitable for IHC on formalin fixed biopsies has shown to be difficult and often results in binders with high background signals and poor specificity [30,31]. In addition, the use of commercially available antibodies has not been adequately validated.
The effect of the formalin fixation during the IHC process on the conformation of protein epitopes is complex; it can leave the native protein conformation unaltered, or can completely destroy conformational protein epitopes. Based on the linear epitope model described by Sompuram et al, the most potent antibodies for IHC recognize linear epitopes that become exposed in unfolded proteins [32-34]. Therefore, to generate binders that could be used for IHC, our approach incorporated filter selections and screening methods designed to identify antibodies that specifically bind to denatured GARP. After 2 or 3 rounds of selection, 50% (pFJ011) and 25% (pFJ014) of the tested individual soluble VHH Fc in periplasmic fractions showed specific binding with high sequence diversity and diverse binding properties. A panel of representative VHH-Fc fusions (based on the difference in their CDR3 region) was selected for further characterization and demonstrated specific binding on ELISA, SPR and western blotting. Flow cytometry analysis on cell surface expressed huGARP showed limited binding for clones 02D05 and 02D07 assuming that those clones likely only bind in denaturing and reducing conditions. Remarkably, an irrelevant cellular binding for clone 01E08 was observed despite an apparent specific binding on WB cell lysates. Finally, two VHH Fc fusions 01G04 and 02D07 were selected and tested for specific GARP staining in human tissue by immunohistochemistry. Both clones showed binding on FFPE embedded GARP transfected cells and in normal tonsil tissue on immune and endothelial cells, known to express GARP. On top, specificity of the staining was determined via RNAscope®, which demonstrated that both clones featured an overall good association between GARP mRNA transcript levels and GARP protein. These results demonstrate that using the pFJ011 and pFJ014 vector systems, clones could be discovered that gave a specific staining pattern for huGARP on FFPE material. Whereas both vector systems can be applicable for different variable domain (VHH, scFv, VH and VL) libraries, VHH’s were chosen for their high stability and solubility. In addition, VHH can very effectively refold after heat denaturation making them the preferred format for diagnostic methods, like immunohistochemistry [35]. Interestingly, using the triple vector, VHH Fc fusions could be directly produced in mammalian cells with high yields, purity and specificity for binding to huGARP.
We created two vector systems, of which the dual vector enabled the display of Fc fused molecule on phage and the triple vector allowed the direct expression in mammalian cells. The combination of these display systems, together with an efficient selection strategy, allowed efficient and rapid identification of anti-GARP VHH Fc clones suitable for immunohistochemistry. To our knowledge, this is the first report describing these features. The next logical step to fully demonstrate the potential of our vector platform is to explore the potential display and mammalian expression of IgG molecules.
Conceptualization, H.d.H. and M.G.P; methodology, G.D.B and I.T.; software, E.P; validation, J.J and B.V.D.W; formal analysis, E.P; investigation, G.D.B and I.T; resources, L.P and R.Q; data curation, G.D.B; writing—original draft preparation, G.D.B and I.T.; writing-review and editing, J.J; visualization, I.T.; supervision, J.J; project administration, G.D.B and I.T; funding acquisition, H.D.H and M.G.P. All authors have read and agreed to the published version of the manuscript.
This research received no external funding
Not applicable.
Not applicable.
Not applicable.
The authors thank Sophie Lucas and Nicolas van Baren at the UCL for reagents, technical advice, and scientific discussions.
GdB, JJ, EP, BVDW, HdH are present or former employees of argenx and IT, LP, MPG, RQ are former or present employees of FairJourney Biologics.


