Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Oumarou Josué Hile1-3, Biyong Issack3,4*, Valère Tala1,2, Ezechiel Ngoufack Jagni Semengue2, Grace Beloumou2, Sandrine Djupsa2, Collins Ambe Chenwi1,2, Alexis Ndjolo1,2 and Joseph Fokam1,2
Received: February 03, 2025; Published: February 14, 2025
*Corresponding author: Biyong Issack, Center de Psychotraumatologie et de Médiation (CPM), Neuchâtel, affiliated @ ISFM: Institut Swiss Institute for Advanced Medical Training Berne, Institute of Psychotraumatology and Mediation, IPM-International, Switzerland
DOI: 10.26717/BJSTR.2025.60.009468
Introduction: The number of people living with HIV in 2017 was estimated at 36.9 million, 21.7 million were
on antiretroviral therapy (ART), an increase of 2.3 million since 2016 and 8 million since 2010. These numbers
underline the incredible progress made in expanding access to antiretroviral treatment (ART) [1]. However, this
expansion is associated with a growing risk of emergence and transmission of HIV drug resistance, leading to
a loss of ART efficacy. In Cameroon in 2017, the prevalence of pre-treatment resistance in naive patients was
7.7%, with evidence of regional variation [2]. Few data exist on pre-treatment resistance in the Far North Region,
hence the need to determine the HIV-1 genotypic resistance profile in patients initiating antiretroviral therapy
in the Far North Region of Cameroon.
Objective: To determine the HIV-1 genotypic resistance profile in patients initiating antiretroviral therapy in the
Far North Region of Cameroon.
Methodology: To achieve our objective, we conducted a cross-sectional analytic study from January 30, 2019, to
May 30, 2019, in urban (Maroua Regional Hospital, Meskine Hospital) and rural (Pétté District Hospital, Tokombéré
Catholic Private Hospital, Mora District Hospital) HIV care centers in the Far North Region of Cameroon.
After verification of inclusion criteria, ART-naive patients with informed consent were enrolled. For each patient,
10 ml of blood was collected, aliquoted into plasma and sent to the virology laboratory of the Centre International
de Reference Chantal Biya (CIRCB), where genotypic resistance tests were performed. Recall software version
2.28 was used to analyze the sequences generated. Interpretation of mutations associated with HIV-1 resistance
was performed using Stanford HIVdb software version 8.7; phylogenetic analysis was performed using MEGA
software version 7.0.2.1. Epi Info software version 7.2.2.16 was used for statistical analysis; comparison of proportions
using Fischer and Chi 2 tests with a significance level of 5%.
Result: Of the 91 patients approached, 86 were selected, of whom 59 extractions were performed. Polymerase
chain reaction (PCR) was performed on 47 RNAs, giving an amplification rate of 80% and a sequencing rate of
100% (40 out of 40 sequences). The study population was predominantly female, with an average age of 35
+/- 9.8 years, 72% of participants were enrolled in urban areas versus 28% in rural areas, and 84.1% were
classified as WHO Stage I. The overall threshold for pre-treatment resistance was 20.0% (95% CI: 9.0; 36.6),
with 25.0% (95% CI: 9.7; 46.7) in urban areas and 12.5% (95% CI: 1.5; 38.3) in rural areas. The prevalence
of pre-treatment resistance to first-generation non- nucleoside reverse transcriptase inhibitors (NNRTIs) was
15.0% (95% CI: 5.7; 29.8), with a prevalence of 16.6% (95% CI: 4.7; 37.3) in urban areas and 12.5% (95% CI: 1.5;
38.3) in rural areas. With Nucleoside Reverse Transcriptase Inhibitors (NRTIs), the prevalence of pre-treatment
resistance was 2.5% (95% CI: 0.0; 13.1), as with Protease Inhibitors (PI/r). NNRTI-associated mutations were
mainly: K103N (10%), Y188L/F (7.5%). The NRTI-associated mutation was M184MV, and the PI/r-associated
mutation was M46I. Genetic diversity was significant, with nine viral subtypes including six recombinant forms
(CRF02_AG, CRF11_cpx, CRF45_cpx, CRF02_AG/A1, F2/G, CRF011_cpx) and three pure subtypes (G, A1, F2). Notably,
CRF02_AG was the most recurrent strain (47.5%), and above all two potential new strains were discovered,
the recombinant forms CRF02_AG/A1 and F2/G. The risk of pre-treatment resistance was higher in patients living
in urban areas and in patients infected with recombinant CRF02_AG forms; OR 2.3 (95% CI :0.4 ;13.3), p=0.3
and OR=2.6 (95% CI :0.4 ;15.1), p=0.2 respectively. We found no significant association between pre-treatment
resistance and gender OR=0.8 (95% CI :0.1 ;5.4), p=1.0; clinical stage OR=0.7 (95% CI :0.0 ;43.3); p=1.0, subtype
OR=1(95% CI :0.1 ;6.2); p=1.0 and age (p=0.2).
Conclusion: This study shows that the overall threshold of HIV-1 pre-treatment resistance in ART- naïve patients
in the Far North Region of Cameroon is high (above 10%, the critical threshold set by the WHO), with a disparity
between urban and rural areas. A resistance profile dominated by the presence of NNRTI mutations. In the Far
North Region, this alarming rate of resistance means that all patients initiating ART in both urban and rural areas
must start with a NNRTI-free protocol. This requires initiation of therapy based on the viral genotypic profile.
Keywords: HIV-1; Pre-Treatment Resistance; Naive Patient; Genotypic Mutation; Antiretrovirals
Abbreviations: ART: Antiretroviral Treatment; CIRCB: Centre International De Reference Chantal Biya; PCR: Polymerase Chain Reaction; NRTIs: Nucleoside Reverse Transcriptase Inhibitors; NNRTIs: Non- Nucleoside Reverse Transcriptase Inhibitors; ARV: Antiretroviral
HIV, a retrovirus of the lentivirus family, remains a major threat to public health worldwide. In 2017, 36.9 million people were living with HIV, nearly 70% of them in sub-Saharan Africa, making this region the epicenter of the epidemic [1]. West and Central Africa is the second most affected region after East and Southern Africa. Since its emergence, the HIV pandemic has had a considerable impact on populations, with 77.3 million people infected worldwide and 35.4 million deaths recorded [1]. In response to this crisis, UNAIDS launched the 90-90-90 initiative in 2014, aiming to control the HIV epidemic by 2020 [3]. This ambitious goal was for 90% of people living with HIV to know their HIV status, for 90% of those who are aware of their status to have access to antiretroviral (ARV) treatment, and for 90% of those on treatment to achieve an undetectable viral load, thereby reducing the risk of transmission [3]. Although significant progress has been made thanks to this initiative, achieving these goals remains a challenge, particularly in certain regions such as Cameroon. Wider access to antiretrovirals has transformed the care of people living with HIV, leading to improved disease management and reduced mortality. However, resistance to treatment represents a major obstacle to the efficacy of antiretroviral therapy. This resistance may be present before the start of treatment (primary resistance) or arise after a certain period of treatment (acquired resistance), reducing the efficacy of ARVs and increasing the risk of therapeutic failure.
According to WHO recommendations, a resistance rate of over 10% calls for a reassessment of treatment protocols to better meet patients’ needs [4]. In Cameroon, the situation remains complex, with the prevalence of ARV resistance varying from region to region, reaching rates of 16.6% in the south-west of the country [2]. This variability underlines the importance of conducting region-specific studies to collect up-to-date data on resistance, particularly in the Far North where information is often limited. These data are essential for adjusting care strategies, improving treatment management and reinforcing efforts to reach global targets in the fight against HIV.
General Objective
Assessing HIV-1 genotypic resistance profile in patients initiating antiretroviral therapy in the Far North Region of Cameroon.
Specific Objectives
The main objective of this study is to assess the genotypic resistance profile of HIV-1 in patients initiating antiretroviral therapy in the Far North Region of Cameroon. This assessment will provide a better understanding of the prevalence of resistance prior to treatment initiation and identify specific mutations that compromise antiretroviral efficacy. In particular, the study aims to determine the global threshold of resistance in this region, compare resistance rates between urban and rural areas, and analyze mutations in relation to different drug classes. It also explores circulating viral subtypes and their potential impact on resistance, as well as clinical and demographic factors associated with resistance, to better adapt treatment strategies.
The methodology of this study is based on an analytical cross-sectional approach conducted in several HIV care centers located in the Far North region of Cameroon. The study was spread over a fourmonth period, from January 30 to May 30, 2019, and included both urban (Maroua Regional Hospital, Meskine Hospital) and rural centers (Pétté District Hospital, Tokombéré Private Catholic Hospital, Mora District Hospital), thus enabling a comparison between urban and rural settings.
• Selection of Participants
Antiretroviral therapy (ART)-naive patients, i.e. those who had never received antiretroviral therapy before, were selected after verification of specific inclusion criteria. These criteria ensured that only eligible patients participated in the study. In addition, each patient gave informed consent prior to enrolment, ensuring that their participation was voluntary and that they were informed of the study’s objectives and procedures.
• Sample Collection and Analysis
For each participant, a 10 ml blood sample was taken, then aliquoted into plasma for subsequent testing. The samples were then sent to the virology laboratory of the Centre International de Référence Chantal Biya (CIRCB), located in Cameroon’s capital, Yaoundé. This laboratory specializes in virological analysis and performs genotypic resistance tests, which are essential for identifying mutations in the HIV genome that could cause resistance to antiretroviral drugs.
• Genetic and Statistical Analysis
a) Genetic Sequence Analysis: Sequences generated during resistance testing were analyzed using Recall software (version 2.28). This software ensures sequence quality and identifies specific mutations in the virus genome.
b) Mutation Interpretation: Mutations associated with antiretroviral resistance were interpreted using Stanford HIVdb software (version 8.7). This software is a recognized database for the analysis and interpretation of HIV resistance to the various treatments available, facilitating decision-making for appropriate treatment.
c) Phylogenetic Analysis: A phylogenetic analysis was also carried out using MEGA software (version 7.0.2.1) to examine the evolutionary relationship between different strains of HIV-1. This enables us to understand the transmission and genetic diversity of the virus within the population studied.
• Statistical Analysis
The data collected were statistically analyzed using Epi Info software (version 7.2.2.16). This software is widely used in the public health field to analyze epidemiological data. Proportions between different variables were compared using Fischer and Chi² tests, with a significance threshold set at 5%. This threshold indicates that differences observed between groups are considered statistically significant if the probability of error is less than 5%. In short, this rigorous methodology makes it possible to obtain reliable and precise results concerning resistance to antiretroviral treatments in the Far North region of Cameroon. The use of appropriate tools and software guarantees in-depth, detailed analysis of the data collected.
A total of 91 patients were approached for the study, and 86 patients gave their informed consent. Of the 05 patients who were not included, 04 refused to participate, 1 was in treatment re-initiation.
General Characteristics of the Study Population
a. Population Distribution by Gender and Age Group
Of the 86 participants selected for the study, 49 (57%) were women and 37 (43%) men (Figure 1), a female/male sex ratio of 4/3. The mean age was 35 +/- 9.8, with extremes of 20 and 64 years, and the most represented age group was [30-39 years] (Figure 2).
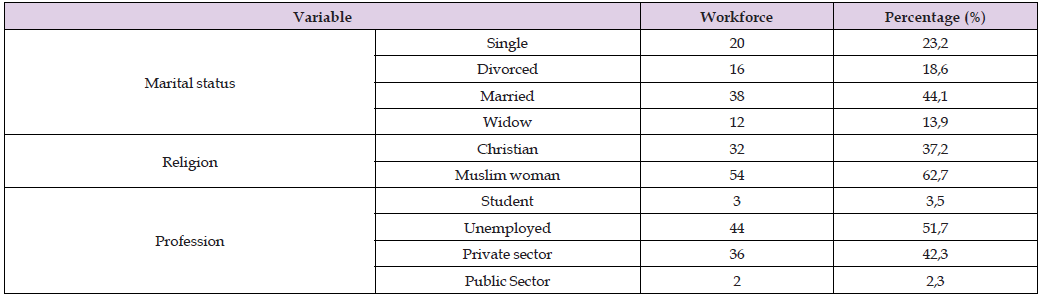
b. Population Distribution by Marital Status, Religion and Occupation
Most of the participants were married (44.1%), and Muslims accounted for 62.7% of the study population. The majority of participants were unemployed (51.7%) (Table 1).
Table 1: The following table summarizes the distribution of the study population according to marital status, religion and profession.
c. Distribution by Clinical Status
Figure 3 summarizes the distribution of the study population according to clinical stage.
Most participants in our study were at time of enrolment in WHO clinical stage I (84%) (Figure 3).
d. Population Distribution by Area of Residence and Level of Education
Figures 4A & 4B show the distribution of the study population area of residence and level of education.
In our study population, 72% of patients were from urban areas and 28% from rural areas, most of whom (54.1%) had no formal education (Figure 4).
HIV Resistance and Genetic Diversity in Far North Region of Cameroon
Global Threshold of Hiv-1 Pre-Treatment Resistance in Far North Region: Considering mutations leading to ineffectiveness with at least one ARV, we found eight patients with resistance mutations out of the 40 samples, giving an overall prevalence of pre-treatment resistance of 20.0% (95% CI: 9.0; 36.6). The prevalence of pre-treatment resistance to first-generation NNRTIs was 15. (95% CI: 5.7; 29.8) and 5.0% (95% CI: 0.6; 16.9) to second-generation NNRTIs. The prevalence of pre-treatment resistance to NRTIs and PI/r was the same, i.e. 2.5 (IC 95%: 0.0; 13.1).
Hiv-1 Pre-Treatment Resistance Threshold Between Urban and Rural Localities: In urban areas, the prevalence of pre-treatment resistance was 25.0% (95% CI: 9.7; 46.7), with first- generation NNRTI resistance at 16.6% (95% CI: 4.7; 37.3). In rural areas, the prevalence of resistance to first-generation NNRTIs (12.5% (95% CI: 1.5; 38.3)) represented the prevalence of pre-treatment resistance.
Mutation Profile: Figure 5 shows the distribution of mutations encountered by ARV class according to their distribution in our study population. NNRTI were in the majority, with the K103N mutation () the most common, followed by Y188L/F (7.5%) and other mutations present at non-relevant rates (<5%) (Figure 5).
Genetic Diversity: Nine viral strains were found, with a large majority of the recombinant form CRF02_AG (47.5%) (Table 2). Figure 6 shows the phylogenetic tree of HIV sequences and reference subtypes. Genetic evolution analyses were performed using MEGA software Version 7.0.2.1. Analysis was based on 54 nucleotide sequences from the HIV-1 protease-reverse transcriptase regions, including 40 patient sequences. Subtypes were assigned to a sequence when the percentage of identity with the reference sequence was ≥ 70%.
Note: (*) CRF02_AG/A1 and F2/G represent potential virus strains discovered from the pol sequence.
• Factors Possibly Associated with Pre-Treatment Resistance
Distribution of pre-treatment resistance by gender, age group, area of residence, clinical stage, recombinant form and Women were more likely to have pre-treatment resistance than men, however there was no association between gender and pre-treatment resistance, p=1. The age [20-29], [30-39] were those where pre-treatment resistance was encountered. Patients living in urban areas were twice as likely to be infected with resistant virus than those in rural areas, but there was no association between resistance and area of residence, p=0.33. Asymptomatic patients were less at risk of pre-treatment resistance than symptomatic patients, yet no association was found between pre- treatment resistance and WHO clinical stage, p=1. AG subtypes were about 3 times more likely to have pre-treatment resistance than non-AGs, but there no association between pre-treatment resistance and AG and non-AG forms, p=0.2. Pure subtypes were less at risk of resistance than recombinants, but there was no association between pre-treatment resistance and subtype, p=1 (Table 3).
Our study aimed to determine the genotypic resistance profile of HIV-1 in patients initiating antiretroviral therapy in the Far North Region of Cameroon. It provided a better understanding of the overall prevalence of pre-treatment resistance, examined differences between urban and rural areas, identified circulating viral subtypes, analyzed the mutational profile by drug class, and highlighted factors associated with pre-treatment resistance. An analytical cross-sectional study was carried out to achieve these objectives, with 94.8% of the 91 patients approached participating. Demographically, women represented 57% of the sample, a proportion like that observed in other studies in sub-Saharan Africa, notably in Cameroon [2]. This trend may be explained by women’s greater vulnerability to infection, due to biological and social factors. In terms of geographical distribution, 72% of patients lived in urban areas, in line with results obtained in other regions of Cameroon [2]. This urban preponderance could be explained by greater population density and more frequent access to health services in towns. Furthermore, most patients had little or no schooling, highlighting the challenges of access to education, particularly in this region of Cameroon. The high rate of unemployed patients (51.75%) also underlines the socio-economic precariousness of this population, a factor that could exacerbate vulnerability to infection.
Clinically, most patients were in the early stages of the disease (stage I and II of the WHO classification), reflecting the increasing effectiveness of early detection campaigns thanks to efforts to achieve the global objectives of the 90-90-90 strategy [5]. In terms of genotypic resistance, our study revealed an overall prevalence of pre-treatment resistance of 20.0%, classified by the WHO as high [6]. This rate is comparable to that observed in other regions of Cameroon, notably the West, where recent studies have reported similar prevalences [7]. Pre-treatment resistance was more frequent in urban areas (25.0%) than in rural areas (12.5%), confirming the trend observed in other national studies [7]. This disparity could be explained by greater exposure to antiretroviral treatments in urban areas, leading to higher selective pressure and therefore a greater risk of resistance. Resistance mutations were dominated by those to first-generation NNRTIs, a class of drugs widely used in first-line treatment in Cameroon [8]. Furthermore, analysis of viral subtypes showed a high prevalence of the recombinant CRF02_AG form, which is consistent with data available in the region and in other Central African countries. The discovery of two new potential recombinant strains (CRF02_AG/A1 and F2/G) testifies to the genetic diversity of HIV-1 in this region. However, no significant association was found between viral subtype and pre-treatment resistance. Despite the robustness of these results, our study has certain limitations.
Sanger sequencing, although the standard clinical method, only analyzes the majority viral population, thus omitting minority viral populations. In addition, the absence of data on the chronicity of infection and the immunological status (CD4) of patients limits our ability to establish more precise links between resistance and these factors. Finally, we encountered logistical and organizational difficulties, including variable buy-in from medical staff, difficult access to some sites, and cold chain constraints for sample transport. These challenges illustrate the need to strengthen local infrastructure and capacity to conduct similar studies in the future.
At the end of this study of HIV-1 genotypic resistance in patients initiating antiretroviral therapy in the Far North region of Cameroon, it was observed that the overall threshold of HIV-1 pre-treatment resistance is particularly high, reaching 20%, which contradicts the initial hypothesis. This pre-treatment resistance varied significantly between urban and rural areas, with a higher prevalence in urban areas. However, in both types of localities, prevalence exceeds the WHO threshold, revealing the scale of the problem in this region. In terms of mutations, resistance is mainly linked to first-generation NNRTIs, which are part of the first- line protocols used in Cameroon. Furthermore, a high level of HIV-1 genetic diversity has been observed in this region, with a strong predominance of the CRF02_AG subtype and the discovery of two potential new recombinant strains (CRF02_AG/A1 and F2/G). This diversity could influence the evolution of resistance to treatments. In addition, factors such as infection with the recombinant CRF02_AG subtype and residence in urban areas appear to increase the risk of pre-treatment resistance to HIV-1, underlining the need to adapt management strategies in these specific contexts to better control the spread of the virus.
We recommend that in future studies focused on this population in the Far North of Cameroon or elsewhere, with HIV-1 treatment residencies, that assessments of HIV-associated neurocognitive disorders and psychiatric disorders be considered. People in psychosocial distress faced with the announcement of the resistance of their condition with residency to existing treatments graciously made available at no cost to them would be very detrimental to their mental health with massive neurocognitive and psychiatric disorders in the medium and long term compared to populations with access to successful treatment without resistance. A MoCA validation study of neurocognitive disorders in this population would be relevant, as was done in adolescents in Yaoundé, Cameroon [9] in 2024 at the Yaoundé University Hospital and the Chantal Biya Foundation.


