Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sajjad Sadeghi1,2*
Received: January 07, 2024; Published: January 10, 2025
*Corresponding author: Sajjad Sadeghi, Department of forensic toxicology, Legal Medicine Research Center, Legal Medicine Organization, Tehran, Iran
DOI: 10.26717/BJSTR.2025.60.009420
Background: The global weight loss supplements market has expanded due to increased health consciousness.
However, there’s a worrying trend of fake herbal supplements containing undisclosed pharmaceuticals like
methamphetamine, which poses serious health risks. This highlights the need for stricter quality control and
regulatory oversight.
Methods: I purchased a package of 50 capsules called “Alpha Slim” from an online herbal shop in Iran. Since the
capsules are not registered with the Iranian Food and Drug Administration, I had 10 of them analyzed for any
undeclared active pharmaceutical ingredients (APIs) at the Forensic Toxicology Laboratory using gas chromatography-
mass spectrometry (GC-MS) and other validated techniques.
Results: The study discovered undisclosed methamphetamine in herbal supplements labeled ALPHA SLIM, using
gas chromatography-mass spectrometry. The supplements contained methamphetamine despite it not being
listed as an ingredient, with a methamphetamine concentration of 12122 ± 100.807 μg/capsule.
Conclusions: The discovery of undisclosed methamphetamine in herbal weight loss supplements requires immediate
action to strengthen quality control measures, promote transparency, and educate consumers to protect
public health and ensure the safety of individuals seeking natural weight loss options.
Keywords: Weight Loss Supplements; Undisclosed Pharmaceuticals; Methamphetamine; Quality Control; Consumer Safety
The global market for weight loss supplements has witnessed exponential growth, fueled by increasing health consciousness and the desire to achieve optimal body weight [1-6]. Research conducted among regularly exercising Turkish women revealed that 44.8% of participants used herbal dietary interventions for weight loss, with green tea being the most commonly used supplement [7]. In Central Mexico, a high prevalence of 45.2% was reported for the concomitant use of herbal products for weight loss and allopathic medicine, leading to adverse reactions and affecting medication adherence [8]. Among the myriad of weight loss products available, herbal supplements have gained popularity due to their perceived natural origins and minimal side effects [9-15]. However, a disturbing trend has emerged one that threatens both consumer safety and the credibility of the dietary supplement industry [16-19]. The counterfeiting of herbal weight loss supplements with undisclosed pharmaceuticals is a troubling matter that, numerous studies have underscored the detection of compounds such as sibutramine, fluoxetine, and ephedrine in these products [20-23]. The presence of undeclared substances in herbal supplements presents significant health hazards, underscoring the necessity for rigorous quality control and regulatory supervision in the production and sale of these products to safeguard consumer health [24]. Methamphetamine, an illicit substance, is a potent psychostimulant derived from amphetamine, leading to considerable somatic, psychiatric, and cognitive complications [25]. Chronic use of methamphetamine may lead to impaired cognitive functions, compromised decision-making, and diminished psychomotor abilities, which can result in delusions and psychotic [26,27].
Undeclared methamphetamine has been detected in herbal weight loss supplements [28]. cases of overdose and toxicity from herbal supplements containing methamphetamine-like compounds have been reported [29-31]. A patient intoxicated by a herbal stimulant containing para-methylthioamphetamine [31]. Moreover, reports of individuals developing serious conditions like Morgellon’s disease from methamphetamine use [32]. a 41-year-old male experiencing various health issues due to methamphetamine abuse [33]. No lethal case of methamphetamine reported in herbal supplement [33]. In this critical examination, we delve into the complexities surrounding the presence of undeclared methamphetamine in weight loss herbal supplements. We explore the reasons behind this adulteration, the challenges faced by regulatory agencies, and the analytical methods employed to detect such substances. Additionally, we discuss the implications for public health and the urgent need for greater transparency and quality control within the dietary supplement industry. The case study of “Alpha Slim,” a popular weight loss herbal supplement, serves as a focal point for our investigation. By understanding the mechanisms behind methamphetamine adulteration and its impact on consumers, we aim to contribute to informed decision-making and promote safer weight loss practices.
Chemicals
The standard for d, l-methamphetamine hydrochloride was obtained from Lipomed Pharmaceutical in Arlesheim, Switzerland, and helium gas of 99.999% purity was obtained from Faransanat Co in Tehran, Iran. Methanol was purchased from Merck Co. in Darmstadt, Germany. All chemicals and solvents used were of analytical reagent grade.
Sampling and Sample Preparation
A package of 50 capsules, advertised as a weight loss supplement and not registered with the Iranian Food and Drug Administration (IFDA), was obtained from an online herbal shop in Iran. Each red capsule is stamped with the words “Alpha Slim”. Each red capsule is stamped with the words “Alpha Slim”. 10 out of 50 capsules were analyzed by the Forensic Toxicology Laboratory to detect undeclared API according to my previous publications. All capsules were analyzed by the Forensic Toxicology Laboratory to detect undeclared API in accordance with my previous publications [2,22]. Organoleptic characteristics, including sample weight, odor, and color, were documented for each capsule. For drug extraction, we employed a straightforward liquid-liquid extraction (LLE) method: 1 mg of the sample was combined with 3 ml of methanol and agitated for 20 minutes in a test tube using a rotator. Subsequently, the mixture underwent centrifugation for 3 minutes at 3000 rpm. The supernatants were then filtered through a PTFE syringe filter (Macherey-Nagel, Germany) and collected. For qualitative analysis, we injected the extracted samples into a GC-MS, with an injection volume of 0.2 μL in splitless mode [34].
Method Validation Procedures
Systematic toxicological analysis of all samples was performed using validated gas chromatography-mass spectrometry (GC-MS) techniques [35]. A gas chromatograph (GC, model 7890B, Agilent, USA) equipped with a split/splitless injector was used together with an HP- 5-MS capillary column (30m length × 0.25 mm ID × 0.25μm film thickness, composed of 5% phenyl silicone and 95% dimethyl polysiloxane). The GC settings were as follows: the injector temperature was set at 280°C, the transfer line temperature at 310 °C and the initial column oven temperature at 80 °C, which was held for 1 minute. The oven temperature was programmed to increase at a rate of 10 °C/min to a final temperature of 300°C, with a final hold time of 22 minutes, for a total of 45 minutes. A constant flow rate of 1.2 ml/min was maintained for the helium carrier gas, which had a purity of 99.99%. The mass analyzer (model 5977B, quadrupole, Agilent, USA) was coupled to the column and operated by electron impact at 70 eV in a positive full scan mode from 50 to 550 m/z. Libraries from the National Institute of Standards and Technology (NIST-2014), Maurer/Pfleger Weber (MPW; 2011) and Wiley (2011) were used for the identification of undeclared active pharmaceutical ingredients (APIs).
According to my previous publications, the GC/MS method for the detection of many drugs has been pre-validated in the forensic laboratory [2,22]. Sample preparation and instrumental conditions were standardized as a general approach to the detection of drugs with natural, acidic, and basic properties. The linearity of the method was evaluated by plotting a calibration curve for sibutramine. The resulting graph showed the peak area of the analyte over five different concentrations, each tested in triplicate. Regression analysis was applied to the data, which fit the equation y = ax + b. The sensitivity of the method was assessed by determining the limit of detection (LOD) and limit of quantification (LOQ) based on the standard deviation (SD) of ten injections at a low concentration of sibutramine (see Table 1). Methamphetamine was used as the internal standard for quantitative analysis. Calibration was performed using five-point calibrators, each measured in triplicate at concentrations of 400, 800, 1600, 3200, and 6400 ng/ml. The regression line, plotted using the method of least squares, gave a correlation coefficient (R^2 = 0.9990) indicating the relationship between the area under the curve (AUC) and the concentration of sibutramine.
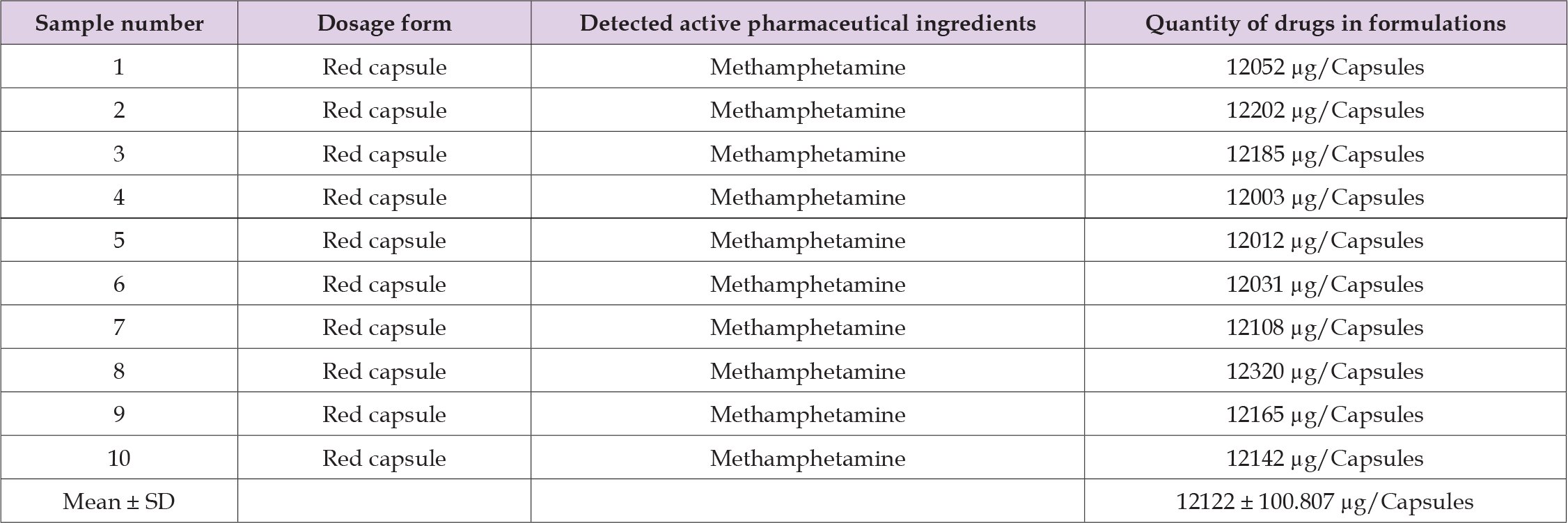
The current study revealed the presence of undeclared methamphetamine in herbal supplements, identified using liquid-liquid extraction (LLE) and gas chromatography-mass spectrometry (GC-MS). The examined sachet of herbal supplements bore the manufacturer’s name (ALPHA ALIM), with a production date of January 2020 and an expiration date of January 2023. However, it lacked a product number and batch number. The ingredient list included Asphatlu 40 mg, Vidanga 25 mg, Piper kourroa 30 mg, Garainiya 45 mg, Caffeine 75 mg, and Green Caffeine 35 mg. Methamphetamine was not listed on the label. The red capsules emitted an herbal scent, were white, labeled ALPHA SLIM, and had an average weight of 245.021 ± 0.312 mg (mean ± SD), as depicted in (Figures 1 & 2). Quantitative analysis revealed that all samples contained methamphetamine. The compound’s presence was confirmed through matching retention times (11.08 min) and spectra (base peak at 58.1 m/z) with standard references. For definitive identification, ion ratios of the unknown samples were compared with those of the standards. The capsules were quantitatively analyzed, indicating a methamphetamine concentration of 12122 ± 100.807 μg/Capsules. (Refer to Figures 3 & 4, and Table 1). The results of the method validation are presented in Table 2 and Figure 5.
Table 2: Sample numbers and active pharmaceutical ingredients detected in adulterated herbal weight loss drugs.
Methamphetamine, a highly potent stimulant, falls under different drug laws around the world [36-38]. It is a commonly abused drug due to its ability to impair cognitive function, induce euphoria and psychomotor impairment, and lead to risk-taking and violent behavior [39]. Methamphetamine abuse is a growing public health concern in Asia, Australia, America, and parts of Europe [40,41]. Methamphetamine abuse is linked to a range of serious health issues, such as neurotoxicity, cardiovascular problems, and mental health disorders. The process involves the excessive production of neurotransmitters, such as epinephrine, dopamine, and serotonin. This can lead to lasting harm at nerve terminals and through various molecular pathways. In order to create successful prevention and treatment plans, it’s crucial to grasp the intricacies and dangers linked to methamphetamine abuse. The study, with Liquid-liquid extraction (LLE) and gas chromatography-mass spectrometry (GC-MS) method, revealed the presence of undeclared methamphetamine in the examined herbal supplements. This discovery is significant because methamphetamine is a potent central nervous system stimulant associated with various health risks. Although some research papers provided do not specifically mention the detection of methamphetamine in these supplements, they do concentrate on identifying sibutramine, canrenone, caffeine, trimethoxyamphetamine, fluoxetine, and ephedrine as frequent adulterants in herbal weight-loss products [28,42,43]. In our previous study, we reported that trimethoxyamphetamine was counterfeited in a weight loss supplement [22].
The issue of undeclared methamphetamine in herbal weight loss and gain supplements poses significant health risks due to the potential adverse effects of the hidden drug. The reason for adding undisclosed API to herbal supplements is to enhance their effectiveness for consumers [44]. Studies have revealed instances where weight management herbal products contained undeclared active pharmaceutical ingredients such as methamphetamine analogs like N,α-diethyl- phenylethylamine (N,α-DEPEA) [21,22,42]. These substances, when added without disclosure, can lead to serious health consequences, including drug interactions, overdose, and addiction [45]. The presence of undeclared methamphetamine in these supplements not only undermines their natural and safe image but also highlights the need for stringent regulatory measures to ensure consumer safety and prevent such adulteration practices in the herbal supplement industry [46]. Therefore, it is crucial for health authorities to increase awareness and enforce strict regulations to address the complexities surrounding the adulteration of herbal supplements with methamphetamine and other undisclosed pharmaceuticals.
Adulteration in herbal supplements can be attributed to various factors such as the lack of stringent regulations in the supplement industry, the allure of high profits, and the misconception among consumers that “natural” products are inherently safe [23,47-50]. The increasing popularity of plant food supplements has created a lucrative market, making these products susceptible to fraud and adulteration, especially when purchased online. Additionally, the absence of standardized regulations globally allows for inadequate oversight, leading to inconsistencies in product quality and safety. Furthermore, the desire to cut costs and maximize profits may drive some manufacturers to substitute cheaper plant materials or use different plant parts than those recommended, compromising the authenticity and efficacy of the supplements. These factors collectively contribute to the adulteration of herbal supplements, posing significant health risks to consumers who may unknowingly be exposed to harmful substances.
Regulatory agencies face significant challenges regarding counterfeited herbal supplements due to the widespread availability and demand for herbal products, coupled with inadequate quality control mechanisms [51,52]. The availability of herbal products online has made it possible for consumers to purchase herbal remedies without expert guidance, but there are risks involved [9,53,54]. the global market for herbal products is rife with authenticity issues, with a substantial proportion of commercial herbal products found to be adulterated, posing serious threats to consumer safety and well-being [55]. The complexity of herbal medicines, containing complex mixtures of plant components and often unidentified compounds, makes quality assessment difficult, further exacerbating the regulatory hurdles faced by agencies [56]. Additionally, the lack of stringent regulations and quality control measures for dietary supplements and herbal remedies contributes to the challenge of ensuring consistent concentrations of active ingredients and product authenticity, highlighting the urgent need for improved regulatory oversight and pharmacovigilance in the herbal medicine industry [57].
Analytical methods play a crucial role in detecting active pharmaceutical ingredients (API) in counterfeited herbal supplements. Various techniques have been developed to address this issue, including chromatography with different detectors such as fluorescence detection, diode array detection, and mass spectrometry [47,58,59]. Also spectroscopic and spectrometric methods use for detect API in adulterated herbal supplements [60,61]. These methods enable the rapid and reliable identification of synthetic drugs like sildenafil, tadalafil, vardenafil, dapoxetine, sibutramine, and phenolphthalein in adulterated herbal products, ensuring consumer safety and regulatory compliance [62,63]. Additionally, the integration of analysis technology with chemometrics methods like class-modeling, discrimination, and regression further enhances the detection of fraudulent activities in herbal medicines, providing valuable reference points for future research and analysis in this field [64].
The dietary supplement industry poses significant implications for public health due to issues such as contamination, adulteration, and misleading labeling [65-69]. Various guidelines on the safe use of traditional medicines have been published by the European Medicines Agency, WHO, and USFDA [70-73]. The lack of stringent regulations globally, especially in the USA, Asia, and Iran, has led to a surge in unsafe products entering the market, risking consumer health and safety [42,54,74-76]. Before buying herbal medicines, it’s recommended to consult with health professionals due to lack of regulating and oversight in Online herbal shop in Iran [42,54,74-76]. Instances of illegal marketing, hidden toxic ingredients, and mislabeling have been reported, highlighting the urgent need for greater transparency and quality control measures within the industry. The presence of unauthorized substances, inadequate safety evaluations, and poor-quality control practices underscore the necessity for improved regulation, post-market surveillance, and collaboration between consumers, healthcare practitioners, and regulatory authorities to ensure the efficacy, safety, and authenticity of dietary supplements, ultimately safeguarding public health and restoring trust in the industry.
The case study of “Alpha Slim,” a popular weight loss herbal supplement, serves as a focal point for our investigation. By understanding the mechanisms behind methamphetamine adulteration and its impact on consumers, we aim to contribute to informed decision- making and promote safer weight loss practices. Considering the findings, it is recommended that, alongside further research and study, new protocols be established for the regulated production and marketing of herbal drugs, as well as for the avoidance of using unapproved herbal medications.
These findings underscore the need for stricter quality control measures, transparent labeling, and regulatory vigilance in the dietary supplement industry. Consumers deserve accurate information about the products they consume, especially when it comes to potentially harmful substances like methamphetamine.
The detection of undeclared methamphetamine in weight loss herbal supplements demands urgent attention. By enhancing quality control, promoting transparency, and educating consumers, we can safeguard public health and ensure the safety of those seeking natural weight loss solutions.
Here are three potential directions for future research related to the detection of undisclosed methamphetamine in weight loss herbal supplements:
• Explore the short-term and long-term health effects of consuming methamphetamine-containing supplements, including potential cardiovascular, neurological, and psychological risks.
• Evaluate the effectiveness of existing regulatory measures in preventing adulteration.
• Investigate the impact of consumer education on purchasing behavior and supplement safety.
Ethical Considerations
All ethical principles were considered in this article.
Funding
No funding.
Acknowledgements
The authors wish to thank all technical staff of the forensic toxicology laboratory of legal medicine center, Bojnurd-Iran for their invaluable assistance.


