Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Steven D’Orio1, Karim Fahmy2, Mahmoud B Rammal2*, Adel Al-Amodi2 and Ilan Hofmann2
Received: October 30, 2024; Published: November 15, 2024
*Corresponding author: Mahmoud B Rammal, I-MED Pharma, Inc., 7190 Rue Frederick Banting, Saint-Laurent, QC, H4S 2A1, Canada
DOI: 10.26717/BJSTR.2024.59.009308
Purpose: Investigate the efficacy of I-LID ’N LASH® PLUS in alleviating the symptoms of patients with blepharitis.
Methods: Twenty patients (16 males/4 females) who participated in this single-center, interventional, non-randomized
study were instructed to use I-LID ’N LASH® PLUS twice daily for 15 days. At the baseline visit, patients
were directed to fill out a symptomology questionnaire to report on various symptoms that are associated with
blepharitis. In addition, a clinical, slit-lamp evaluation was performed at the baseline visit on the subjects to evaluate
the intensity of commonly encountered blepharitis signs, such as swelling, redness, irritation, and debris.
The subjects reassessed their symptoms after the end of the treatment and an in-office evaluation was repeated
to study the change in the clinically reported signs. The subjects were also invited to complete an end-of-treatment
questionnaire to reflect on their experience utilizing the I-LID ’N LASH® PLUS.
Results: The patient-reported assessment demonstrated that using the I-LID ’N LASH® PLUS for 15 days improved
the subjective ocular symptoms. Statistically significant results were obtained for symptoms related to
eye itching, eye irritation, heavy or puffy eyelids, foreign body sensation, and crusty eyes. The clinical assessment
revealed that signs such as eyelid debris and eyelid irritation were significantly reduced following the use of the
I-LID ’N LASH® PLUS. The end-of-treatment assessment ascertained that 85% of subjects did not experience
crusting in the morning post-treatment, 95% did not have any problems while using the wipes, 70% felt improvements
using the wipe, and 55% of patients said their eyes felt less irritated.
Conclusion: I-LID ’N LASH® PLUS proved useful in reducing the severity of signs and symptoms commonly reported
by blepharitis patients, as demonstrated by patient-reported, clinical, and end-of-treatment assessments.
Keywords: Blepharitis; Demodex; Eyelid Hygiene; Eyelid Cleanser; Tea Tree Oil; I-LID ’N LASH® PLUS
Dry eye disease (DED) is a complex, multi-factorial ocular surface disease that transpires when the tear film of the eye does not provide sufficient hydration to the ocular surface. The International Dry Eye Workshop (TFOS DEWS II) defines DED as an “ocular surface disease characterized by a loss of homeostasis of the tear film and accompanied by ocular signs, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” [1]. DED patients commonly suffer from such symptoms as visual disturbance, eye irritation, stinging, dryness, discomfort, and tear film instability accompanied by ocular inflammation and increased tear film osmolarity [2]. While still not fully understood and generally poorly managed, DED is a prevalent condition impacting a large segment of the population. Indeed, a survey revealed that 37% to 47% of patients encountered by ophthalmologists exhibited signs of blepharitis [3]. DED can contribute to deleterious societal consequences including a marked degradation in the quality of life, lower productivity, and a substantial economic burden for the impacted patients [2,4]. To this point, the exact origins and progression of Dry Eye Disease (DED) remain partially obscure. Extensive research has unveiled a plethora of potential triggers, with key etiological contributors including periocular bacterial infection or Demodex mite infestation. These factors are significantly associated with the emergence of blepharitis a contributing factor of DED [5,6]. Blepharitis refers to an inflammatory condition of the eyelid, manifested through symptoms such as burning sensation, irritation, tearing, photophobia, blurred vision, red eyes, and eyelid margin crusting. Blepharitis is classified based on the anatomic eyelid location from which it originates:
(i) Anterior blepharitis and
(ii) Posterior blepharitis. Anterior blepharitis corresponds to
the inflammation at the outside front edge of the eyelid where the
eyelid attaches.
Anterior blepharitis stems from bacterial (mainly Staphylococcus aureus) or viral (Molluscum contagiosum) origins [7]. Posterior blepharitis is discerned by the inflammation of the inner edge of the eyelid that touches the eyeball and has various causes, such as meibomian gland dysfunction (MGD), infectious or allergic conjunctivitis, and systematic conditions such as acne rosacea [8]. Heavy infestation of Demodex mites has also been linked to both anterior and posterior blepharitis [9]. Marginal (mixed) blepharitis comprises patients who suffer concomitantly from both anterior and posterior blepharitis. Several treatment options can be adopted to address blepharitis depending on the severity of the condition. Proper eyelid hygiene, warm compresses, eyelid massage, and eyelid scrubs constitute the commonly prescribed treatment regimens. In certain cases, a course of medical treatment with topical corticosteroid drops might be prescribed to decrease inflammation in an acute exacerbation [10,11]. However, oral, and topical interventions can carry adverse effects for prolonged use including high interocular pressure, posterior cataract formation, superinfections, etc. [11]. Therefore, despite its basic and long-standing nature, eyelid hygiene is still considered the mainstay of the treatment of blepharitis.
An eyelid hygiene regimen commonly constitutes warm compresses, eyelid massage, and eye scrubs. Eyelid scrubbing consists of gently scrubbing the eyelids with a wet washcloth and detergent such as baby shampoo or a commercially available cleansing product to remove scales and debris accumulated on the lids. Commercial products involving cleansing solutions and scrubs are favored by patients due to their convenience; however, their efficacy is still not fully assessed [11]. Indeed, it is notable that many of the currently existing commercial brands lack scientific evidence and supporting studies to substantiate their efficacy. The wide range of commercial eyelid cleansing products currently vary in formulation but typically contain bacteria- and Demodex-eliminating components, mainly tea tree oil (TTO) [12,13]. TTO is an essential oil obtained from the distillation of leaves and terminal branches of the Australian tea (Melaleuca alternifolia) and has been a centerpiece in many commercial eyelid hygiene products due to its antiseptic, antiviral, antifungal, anti-inflammatory and acaricidal properties [14-17].
The objective of this study is to investigate the efficacy of I-LID ’N LASH® PLUS (Figure 1) in assessing the symptomatic relief of patients suffering from blepharitis. I-LID ’N LASH® PLUS is an eyelid hygiene ocular product that consists of pre-soaked wipes with 5% TTO and is recommended as a daily cleansing solution for lids and lashes to mitigate the build-up of eyelid debris and bacteria as well as to control lid margin Demodex infestation. No previous studies have evaluated the efficacy of this commercial product in the context of blepharitis. The study’s outcomes are based on symptomatic relief assessments reported by patients and clinical signs evaluated in the optometrist’s office before and at the end of the treatment period.
I-LID ’N LASH® PLUS is manufactured by I-MED Pharma, a company based in Montreal, Canada, specializing in dry eye diagnosis and management. I-LID ’N LASH® PLUS contains key ingredients serving a wide range of functions, as shown in Table 1. Twenty patients suffering from blepharitis attending the optometric clinic (Steven D’Orio’s clinic “SD”) were recruited in the current pilot, single-centered, non-randomized, single group, cohort, interventional study. Each participant received a comprehensive written information form and provided their explicit agreement to participate by signing the informed consent document. Among the 20 patients recruited, 16 were males and 4 were females. During their initial visit, patients were provided with a questionnaire designed to assess the severity of their symptoms (Table 2). Additionally, the participants underwent a comprehensive ocular exam to assess signs of eyelid swelling, eyelid debris, and eyelid irritation using an evaluation chart and a designated grading system (Table 3). A demonstration on the proper use of the I-LID ’N LASH® PLUS was provided and participants were instructed to use the I-LID ’N LASH® PLUS twice daily (in the morning and evening) for 15 days. A tracking sheet was also provided to ensure compliance. At the end of the study period (15 days), the baseline (patient self-reported and clinical) assessments were repeated. Also, at the conclusion of the trial, subjects were asked to complete a subjective assessment questionnaire to reflect on their experience using the product and rate its efficacy (Table 4).
Table 2: Patient assessment questionnaire to report on subjective improvement of symptoms before and after using I-LID ’N LASH® PLUS for 15 days.
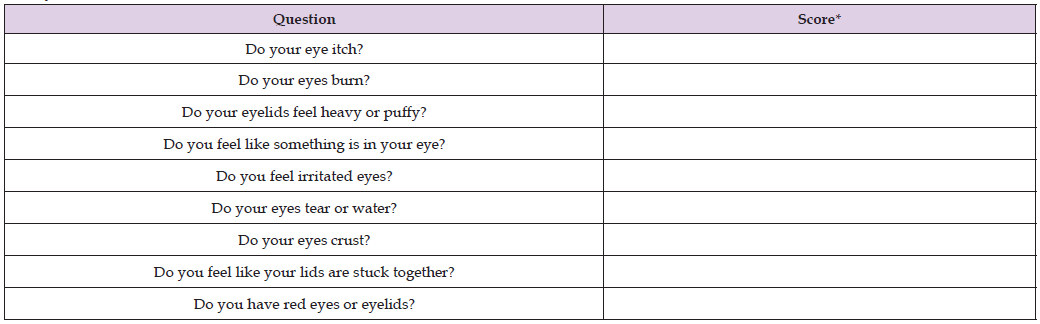
Note: *Score guidance: None of the time (1), Occasionally (2), Frequently (3), or All of the time (4).
Table 3: Clinical evaluation form to assess the severity of commonly frequented blepharitis signs before and after using I-LID ’N LASH® PLUS for 15 days.
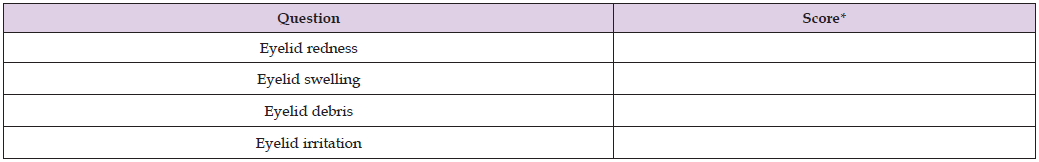
Note: *Score guidance: 0 = None (or no lid/lash debris, or irritation <25% of the time), 1 = Mild (<1/3 of lid/lash has collarettes or debris or irritation 25-50% of the time), 2 = Moderate (> 1/3 of lid/lash has collarettes or debris or irritation 51-75% of the time), 3 = Severe (2/3 of lid/lash has collarettes or debris or irritation >76% of the time)
Table 4: End-of-treatment assessment questionnaire to garner subjects’ feedback after using I-LID ’N LASH® PLUS for 15 days.
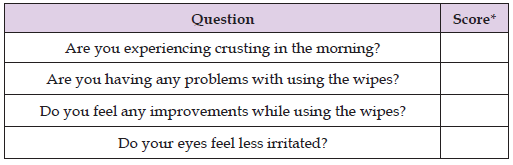
Note: *Score guidance: Yes/No
Statistical Analysis
Statistical analyses were performed using Microsoft Excel (Office 365). A student-paired t-test was utilized to study pre-treatment and post-treatment results. All analyses were two-tailed and p < 0.05 was considered statistically significant.
Patient Assessment
The study was conducted with a total of 20 participants. Throughout the duration of the study, no adverse events were reported, and the product was well-received by all participants, none of whom ceased usage throughout the trial. In general, at baseline, the participants did not exhibit severe symptoms, as evidenced by their subjective ratings which, on average, fell below 2 (2 denotes an occasional frequency of the symptom). The most frequent symptom, albeit mild in intensity, was excessive tearing. Upon employing an eyelid hygiene regimen with I-LID ’N LASH® PLUS for a span of 15 days, participants reported marked alleviation in self-reported symptoms. Notably, statistically significant improvements were observed in several symptoms, including feelings of itchy eyelids (p = 0.028), heavy or puffy eyelids (p = 0.041), a foreign body sensation in the eye (p = 0.015), irritated eyes (p= 0.0051), and crusty eyes (p = 0.028).
Clinical Assessment
Clinical evaluation was performed on the participants at the optometric clinic (SD) to corroborate the symptomology findings obtained from the patient’s assessments. A slit lamp was utilized for this purpose. The clinical assessment showed that signs such as eye redness and eye lid swelling were not prevalent observations at baseline. Indeed, Figure 3 illustrates that most subjects were clinically asymptomatic for eyelid redness with only one patient suffering moderate signs of eyelid redness and similarly only one patient was characterized by mild eyelid swelling. Eyelid debris was the most observed clinical sign with a varying degree of severity, mild (n = 8, 40%), moderate (n = 10, 50%), severe (n = 2, 10%). In contrast, eyelid irritation was a far less common clinical sign with the degree of severity as follows: none (n = 13, 65%), mild (n = 6, 35%), and moderate (n = 1, 5%). After 15 days of applying I-LID ’N LASH® PLUS, there was a statistically significant decrease in both eyelid debris (p = 0.0000073) and eyelid irritation (p = 0.016), indicating a noteworthy improvement in these conditions.
End-of-Treatment Assessment
The end-of-treatment assessment was provided to the subjects to solicit their feedback on the use of the I-LID ’N LASH® PLUS and the corresponding relief from the commonly encountered blepharitis-associated symptoms, as shown in Figure 4. A significant proportion of participants (n = 11, 55%) reported experiencing less eye irritation. The majority of them (n = 17, 85%) indicated an absence of morning crusting following the use of the I-LID ’N LASH® PLUS. In terms of comfort and ease during the application of the lid wipes, an overwhelming of subjects (n = 19, 95%) reported no issues associated with product usage. Of these, a substantial number (n = 14, 75%) reported an actual improvement in their condition while using the wipes.
Chronic blepharitis is a prevalent condition that impacts a large segment of the population of different age groups [7]. While the origins of blepharitis are not widely understood, the standard of care to prevent/treat this ocular condition is based on an eyelid hygiene protocol to reduce the likelihood of bacterial/mite infestation and subsequent inflammation in the eyelid region. The successful treatment of blepharitis is evaluated based on symptomology relief, namely patient-reported improvement in symptoms through pre-defined questionnaires [18]. Additionally, the validation of the symptoms reported by the patients in a clinical setting is vital to corroborate the findings obtained from the patients. That is because there is often a mismatch between the severity of clinical signs and patient-reported signs which convolute both the diagnosis and management of DED in clinical practice. This study aimed to investigate the efficacy of the eyelid hygiene commercial product (I-LID ’N LASH® PLUS) towards the relief of symptoms commonly associated with blepharitis patients using both patient-reported questionnaires and clinical evaluation. The use of I-LID ’N LASH® PLUS led to notable enhancements across all symptoms identified in the patient questionnaire. Also, the prominent signs that are frequently encountered by blepharitis patients (crusting, itching, irritation) were shown to have been alleviated with a statistical significance after implementing the eyelid hygiene regimen with I-LID ’N LASH® PLUS [11]. The relief from blepharitis symptoms can be attributed to the ingredients that are present in the I-LID ’N LASH® PLUS. Primarily, the I-LID ’N LASH® PLUS contains a surfactant (Poloxamer 188) that can aid in removing excess oils from the eyelids, debris, and desquamated skin. This is reasonable since anterior blepharitis has been associated with the overproduction of oil on the preocular surface, whereby bacteria can thrive and lead to the onset of eyelid inflammation [19]. Additionally, the surfactant may enhance the management of blepharitis symptoms, as patients frequently experience greasy eyelids crusted with scales, which is typically challenging to eliminate with a standard surfactant-free cleansing product [11]. Crust, debris, and scales can also interfere with the function of the meibomian glands and exacerbate eye conditions like posterior blepharitis [11]. In addition to the physical removal of crust and debris by the scrubbing action of the wipes and the use of a surfactant, I-LID ’N LASH® PLUS contains TTO which is effective in reducing Demodex infestation and bacterial infection. The preponderance of Demodex and bacteria on the eyelid and lashes have been ascribed to the onset of blepharitis symptoms such as eye irritation, itching, and crusting [11,20].
In fact, in cases where Demodex is the leading cause of blepharitis, a treatment course that can reduce or eradicate eyelid Demodex would principally result in alleviating blepharitis symptoms in patients that would otherwise not benefit from conventional treatment options [21]. While traditional therapy for treating blepharitis has mostly relied on using warm compresses and an antibiotic/steroid combination, these therapies have been deemed futile mostly resulting in the persistence of symptoms, and thus establishing the importance of incorporating an anti-demodex agent like TTO [20]. Demodex is believed to cause eyelid inflammation (blepharitis) since those mites can activate the host immune system and feed off the lipids in the glands, compromising the lipid layer of the tear film; the mites are also thought to serve as hosts for bacteria on their surface such as Streptococci and Staphylococci which are contributing elements to blepharitis [20]. Once dead, mites release the embedded bacteria eliciting inflammation. The presence of cylindrical dandruff (CD) on the base of the lashes is a pathognomonic sign of infestation, those CDs contain proteases and lipases that cause irritation. Demodex-related eyelid irritation can also be triggered by the biting and crawling of the mites on the skin [20].
Additionally, Demodex mites are also implicated in posterior blepharitis as the accumulation of parasite waste can result in the blockage of the gland and a cell-mediated immune reaction. The most effective treatment protocol to manage the Demodex population in eyelids consists of using TTO. Studies have demonstrated that a TTO concentration as low as 5% (when applied to the lids twice daily) is detrimental to mites [22]. The mode of action of TTO against Demodex is not well understood, but it has been hypothesized that terpinene-4-ol, a major component of TTO, is the most active ingredient to kill Demodex [23]. The killing mechanism of TTO has been attributed to its effectiveness in driving the Demodex to exit from the hair follicles, thereby facilitating its elimination by competitively inhibiting the enzyme acetylcholinesterase [24-27]. Additionally, TTO exhibits bactericidal properties, notably against Staphylococcus and Streptococcus whose presence in the preocular region, as previously mentioned, is a primary etiological factor in blepharitis. Furthermore, TTO has an anti-inflammatory feature evidenced by its inherent ability to suppress monocyte-derived pro-inflammatory proteins and oxygen-derived reactive species, thereby contributing to the alleviation of blepharitis-associated symptoms for patients, as shown in this study. The results here are in line with other findings that reported an improvement in subjective ocular symptoms after employing TTObased eyelid wipes for blepharitis patients [13,14,16-18]. While effective, the presence of TTO in eyelid scrubbing products has been reported in some cases to trigger skin reaction, dermatitis, allergy, and ocular irritation, when used at high concentrations [26]. This might lead to poor patient compliance thereby impacting the efficacy of the prescribed treatment. The end of treatment assessment included in this study aimed to measure the patient’s receptivity to using I-LID ’N LASH® PLUS to ensure compliance and long-term use. Only 5% of participants (n = 1) signaled an issue while using the product, which together with the fact that no adverse effect or patient withdrawal occurred during the study, point to the efficacy and patient-friendly nature of the product. The absence of patient-reported discomfort while using the product can be attributed to the relatively low concentration of TTO present in the formulation. While effective against Demodex as part of a regular eyelid hygiene regimen as demonstrated by several in-vitro and in-vivo studies [9,20,27], 5% of TTO has been shown to cause minimal irritation when tested on animals [28]. Additionally, the presence of a humectant like sodium hyaluronate (hyaluronic acid, HA) in the formulation of LID ’N LASH® PLUS imparts a hydration effect on the skin and helps counteract the irritation that could originate from TTO. That is in addition to HA’s anti-inflammatory and wound-healing properties that would further enhance the effective and convenient use of I-LID ’N LASH® PLUS [29].
One limitation of this study relates to the mild-to-moderate severity of the symptoms reported by the patients and the clinical signs evaluated in the optometrist’s office. Also, since this was a preliminary, proof-of- concept study evaluating the efficacy of I-LID ’N LASH® PLUS, the patients were not masked to the treatment used and their symptomology assessment might have been prone to bias, especially in the light of the absence of a control group. It would be beneficial in future studies to follow a double-masked study, with inclusion criteria that include participants with more severe symptoms, while also incorporating a placebo eyelid wipe that does not contain an active bactericidal and anti-mite component. This will shed light on whether the relief from symptoms is due to the mechanical scrubbing or the active ingredients present in the cleansing solution.
Another consideration that might be added to a future study relates to investigating the change in the population of Demodex and the microbiome in the lid margin to ascertain the mode of action of the I-LID ’N LASH® PLUS. Additionally, it is worth noting that the duration of the study was limited to 15 days, a period that might not be sufficient to establish the full effect of TTO. This is because Demodex mites exhibit a life cycle of 2-3 weeks and an eyelid hygiene regimen that aims to reduce or eradicate Demodex must at least cover 2 cycles to eliminate not only adult mites but also any resulting eggs and larvae [20,24].
This study has demonstrated that adopting an eyelid-hygiene regimen using I-LID ’N LASH® PLUS for 15 days contributed to the reduction in blepharitis-associated symptoms as assessed by patient- reported questionnaires and clinical evaluation. Also, evidenced by an end-of-treatment survey, the subjects reported positive feedback on the efficacy and ease of use of the product. While statistically significant results were obtained supporting the efficacy of the product in treating blepharitis symptoms, future studies are warranted to increase the size of the participant pool, refine the inclusion criteria to include patients with more severe symptoms, extend the study duration, implement a randomized design with a parallel placebo group and adopt a double-masked approach to further corroborate the preliminary findings of this study.
Steven D’Orio: Investigation, Writing - Review & Editing; Karim Fahmy: Conceptualization, Methodology, Resources, Supervision, Project administration, Writing - Review & Editing; Mahmoud Rammal: Conceptualization, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization; Adel Al-Amodi: Conceptualization, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization; Ilan Hofmann: Conceptualization, Methodology, Resources, Supervision, Project administration, Writing- Review & Editing.
This study was a collaboration work between I-MED Pharma and the clinic of Dr. D’Orio Eyecare + Associates. I-MED Pharma provided the materials used in the clinical study.


