Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Bekele Lema1*, Mintesnot Tsegaye2 and Ansha Yimer2
Received: September 25, 2024; Published: October 09,2024
*Corresponding author: Bekele Lema, Animal Health Institute, Sebeta, Ethiopia
DOI: 10.26717/BJSTR.2024.59.009232
A promising strategy for managing tsetse flies, which are the carriers of African trypanosome diseases, is the sterile insect technique (SIT). Just sterile males should be released, so successful sex separation is essential to the success of SIT. Prior to their dispersal, adult tsetse flies must typically be chilled for up to an hour at temperatures as low as +4°C through handling, sex sorting, packaging, and aerial release techniques. Tsetse fly handling and transportation using chilling seems to be a workable approach for sterile insect technique programs, despite the fact that it can have certain unfavorable effects. To successfully control the intended wild population, it is crucial to assess the effects of chilling during the sex sorting and handling of both male and female flies. As a result, this study was conducted to ascertain the ideal chilling time as well as the relative impact of chilling time on the rate of survival and reproductive efficiency of both male and female Glossina pallidipes flies raised and maintained in the Kality Tsetse fly Research Center under current laboratory conditions. In order to examine how chilling at 4˚C affects the reproductive and survival of G. pallidipes flies, 160 randomly chosen adult teneral flies were chilled at 4˚C once for 5, 10, and 25 minutes, and the results were compared with a control group of no chilled flies. After being chilled, the reproductive effectiveness and survival of day-old post-chilled flies were tracked for 90 days and contrasted with non-chillied flies. The results showed that the survival rate of G. pallidipes flies was not affected by the length of chilling (P=0.57).
However, after being chilled for 5, 10, and 25 minutes, 76, 71, and 65 percent of the flies managed to survive. In a similar vein, after 90 days, 70% of the control group of non-chilled flies survived. The findings demonstrated that, in comparison to non-chilled flies (control), all reproductive parameters—with the exception of the inter larval period—were significantly impacted by the chilling temperature at 4 °C for exposure times of 25 and ten minutes. Fertility, pupae production, PPIF, and pupal weight were all significantly reduced, but the date of the first larval deposition increased as exposure duration increased. However, in terms of pupal and PPIF, there was no discernible difference between the five-minute chilling exposure and the control group. After taking into account all the variables, it is possible to determine that adult G. pallidipe flies can be handled and immobilized for five minutes at a chilling temperature of 4 °C without causing any degradation in their quality.
Keywords: Chilling Duration; First Larvi-Position; G. Pallidipes; Pupae Per Initial of Female; Sterile Insect Techniques
Abbreviations: NWS: New World Screwworm; SIT: Sterile Insect Technique; CTmin: Critical Thermal Minimum; RH: Relative Humidity; KTFRC: Kality Tsetse Fly Research Center; PPIF: Pupae Per Initial Female; FLPD: First Larvi Position Date; ILP: Inter Larval Period
The genus Glossina and family Glossinidae contain the medically and commercially significant tsetse flies (Diptera: Glossinidae) [1]. Although the two species are found in southwest Saudi Arabia, they are nearly entirely restricted to the sub-Saharan African continent, which lies between 5°N and 20°S in latitude [2]. The southern, southwest, and northwest regions of Ethiopia, between longitudes of 33° and 38°E and latitudes of 5° and 12°N, are the typical distribution areas for tsetse flies [3]. Additionally, as the massifs and plateau of central Ethiopia are carved out of long, steep river valleys, the flies have gradually expanded into these areas [4]. Tsetse flies and parasites are thought to be a major contributor to poverty in Africa, where they cause nagana, or sleeping sickness, in animals and humans [5,6]. Alternative vector control tools are desperately needed, as resistance to traditional vector control methods like insecticides builds up in wild populations [2]. One essential and effective strategy for trypanosomosis control in an area-wide integrated tsetse management approach (AW-ITM) is the use of sterile insects. The method has proven successful in eliminating the tsetse (G. bryani) and new world screwworm (NWS) (Cochliomyia hominivorax) from North and Central America to Panama ten). austeni) from Zanzibar's Unguja Island [7]. The sterile insect technique (SIT) involves transporting sterile males from the mass-rearing facility to the target site, as well as mass-rearing the target species in a lab and subjecting them to a specified dose of gamma radiation to induce sexual sterility [8].
The multiple steps involved in those treatments may have an impact on the biological quality of sterile males and females, particularly with regard to productivity and survival rate. A suitable temperature range and duration for immobilization during sex sorting, transportation, handling, and radiation have already been suggested by earlier studies [9]. The critical thermal minimum (CTmin) or knockdown temperature is the point at which an insect loses its ability to respond to gravity and becomes immobile, preventing it from standing or adhering to a surface. This phenomenon occurs at a temperature that is specific to a given species. This comes before a phase that is referred to as a chill-coma, during which there is a reversible loss of movement [10]. Insects have been demonstrated to become stressed when exposed to temperatures outside of their typical range, which lowers their quality and competitiveness [11,12]. Numerous investigations have revealed that the quality control parameters examined in males were impacted by transport, irradiation, and chilling [10,11]. Nevertheless, research on the impact of the length of chilling on the reproductive efficiency of female tsetse flies during sex sorting and mating has not yet been conducted. Therefore, this study has been carried out to determine the optimum chilling duration anect of chilling duration on reproductive efficiency and survival rate of both female and male Glossina pallidipes flies rear and maintain under existing laboratory conditions in Kality Tsetse fly Research Center.
Tsetse Colony Sources
Tsetse flies of G. pallidipes (Diptera: Glossinidae) colony used in this study was obtained from the Kality Tsetse Fly Research Center in Ethiopia's laboratory. This Glossina pallidipes Austen colony was also established in 2000 [13] and originated in the Southern Rift Valley. Since 2000, these G. pallidipes laboratory colonies have been raised in the same conditions as before at the Animal Health Institute of Ethiopia's Kality Tsetse Fly Research Center (formerly known as Southern Tsetse and Trypanomias control). With its own industrial gamma irradiator, the facility houses two enormous rearing modules specifically designed to raise G. pallidipes and G. Fuscipes colony and the facility can produce over 700 000 sterile male flies per week, enough to treat about 7000 km2 at a time. The colony has a capacity of approximately 7 million female flies [8]. The KTFRC maintains its adult colonies at a temperature regime of 24–25 °C and 75–80 percent relative humidity (RH) with a 12-hour light-dark cycle. The pupae are kept at a temperature regime of 25 °C and 85 percent RH. Pupae were housed at 25°C and 80% relative humidity in a climate-controlled chamber [14,15].
Fly Handling and Sex Sorting Procedures
At the Kality Tsetse Fly Research Center (KTFRC), manual tsetse fly handling, sex soring, and mating (zero mating) were conducted in accordance with the FAO/IAEA (2006) Standard Operating Procedures for Mass-Rearing Tsetse Flies [16]. Additionally, pupae used in all experiments (rearing) were created and hatched in accordance with uniform protocols created at the IPCL [16]. Pupae were housed for 25–30 days at 25 °C and 80% relative humidity in a climate-controlled chamber. After emerging from the pupal stage after thirty days, adults are placed in a 20 cm diameter cage with mesh fabric on top and bottom and are then moved to a chiller to reduce their movement. A chest chiller was used to chill flies in cages at a temperature of +4 oC for five minutes. The cold air caused the flies to become immobile, or chill out. The critical thermal minimum (CTmin), also known as the knockdown temperature, is the phenomenon of immobilizing an insect for five minutes at a species-specific temperature of +2 to 4 °C. At this point, the insect loses its ability to respond to gravity and becomes unable to stand or cling to a surface. This comes before a phase that is referred to as a chill-coma, during which there is a reversible loss of movement [10]. Males and females are then visually distinguished after the chilled flies are dumped onto a counting plate inside the chiller. Male adult flies can be easily identified from females based on their morphology thanks to the presence of the hypopygium, a modified 9th abdominal segment that houses the external genital structure. Then In rearing (not during this experiment) mating is done with pre difined sex ratio (1:4) of male to female and a total of 60 flies are loaded in 20 cm diameter cage and manually transfered cages of flies to the rearing and holding room [16].
Fly Rearing and Maintaining Procedures
The FAO/IAEA (2006) Standard Operating Procedures for Mass-Rearing Tsetse Flies [16] were followed in the production of tsetse at the Kality Tsetse Fly Research Center (KTFRC). For adult G. pallidipes tsetse, the KTFRC colony conditions are 23–24 °C and 75–80% relative humidity (RH); for pupae, the conditions are 25 °C and 75% RH. During the 12:12 photo period, low-key lighting was provided by indirect fluorescent tubes or tungsten lamps. Using an invitro feeding system and defibrinated bovine blood obtained aseptically from the Addis Ababa municipal abattoir in Ethiopia, the colony at the KTFRC has been sustained. The blood was stored in an industrial cobalt 60 gamma irradiator at -20 °C and was 96% irradiated with 1 point 2 kGy at the Kality Tsetse Fly Research Center. After the microbiological test, a thirty-day biosay (feeding test) was conducted to assess the blood's nutritional quality. Five blood meals a week were provided to the flies, who were also kept in perfect environmental conditions and under strict supervision. The blood was fed five days a week (Monday, Tuesday, Thursday, Friday, and Saturday) for ten minutes at a time, with each feeding being adjusted to a temperature between 35 and 37 °C. Every fly was fed 24 hours after emerging, and then five times a week on freshly drawn, defibrinated cow blood. The data logger installed in the rearing room was used to monitor and adjust the humidity and temperature in the holding room on a daily basis. Every day, pupae were gathered, and every week, a mortality check was made. These conditions will henceforth be referred to as standard laboratory rearing conditions [13-16].
Experimental Procedures
In order to assess the single exposure effect of chilling during manual sex sorting (at adult emergence) on the survival and repoductive efficiency of both male and female G. pallidipes flies reared and maintained under existing laboratory conditions in Kality Tsetse fly Research Center, the study, which took place from February 8 to June 8, 2022, included 160 randomly selected G. pallidipes (80 females and 80 males). Four groups of experimental flies were formed based on the length of time they were exposed to chilling (4 °C) temperature for five minutes, ten minutes, twenty-five minutes, and non-chilled (control). The experimental flies used in the different experimental groups were randomly selected from the same batch on February 8, 2022, and reared and maintained under similar environmental conditions using standard rearing procedures. These chilling times were selected because, according to research, five minutes is the minimum chilling time needed to immobilize flies for manual sex sorting [10] and because the control experiment showed what reproductive outcomes tsetse flies can achieve when handled without chilling time, ten and twenty-five minutes are the maximum chilling times. The critical thermal minimum (CTmin) or knockdown temperature is the point at which an insect loses its ability to right itself and becomes immobile, unable to stand or cling to a surface, for five minutes at a temperature that is species-specific (+2 to 4 °C) [13]. Twenty replication of a single sex tube containing one male and one female individual, was created for each trial group.
Approximately equal numbers of both sexes of forty flies underwent a chilling at (4 °C) treatment for five minutes, ten minutes, and twenty-five minutes duration at 4 °C. The remaining flies underwent no chilling treatment (control). Adults were sorted manually by aspirating tubes and sex sorting would be done by visualizing their reproductive anatomy. To collect pupae that have been deposited and are small enough to fall through the mesh, each container was set on top of a pot. The flies were kept in similar rooms with ideal environmental conditions and were raised and maintained using comparable feeding, mating, and handling practices according to [17,18]. The data logger installed in the rearing room was used to monitor and control the temperature and humidity in the holding room on a daily basis. The duration of chilling (0, 5, 10, and 25 minutes) at 4 °C temperature during annual handling at adult emergence was the only treatment variation. The effects of one chilling exposure at a temperature of +4 °C were compared to four exposure times (non-chillied, control), 5, 10, and 25 minutes) on a survival of a-day-old newly emerged teneral fles of G. pallidipes (Diptera: Glossinidae) over 120 days. Additionally, we examined the post chilling effect on reproductive efficiency and longevity of adult flies and compared with those that were not chilled over 90 days.
Chilling Effect on Flies Survival: Immediate mortality was assessed following immobilization and their survival was monitored for a further 120 days, with dead adults removed daily. Survival was not monitored beyond 14 days as survival post-chilling is not likely to exceed this timeframe. Mortality was recorded daily for each test group throughout the experimental period. Mortality rate in each cage was checked every day starting from day 1 after emergence up to the end of experimental period. Dead flies were recorded based on their sex, age and cause of mortality (into overchilled, blood-fed, starved fly) mortalities. The mortality rate was calculated according to Standard Operating Procedures for Mass-Rearing of Tsetse flies. Survival was calculated by subtracting the number of dead flies from the previous day recorded of the total number of survival flies in each cage [16,19].
Chilling Effect on Reproductive Efficiency of Flies:
Pupae Per Initial Female and Pupae Quality: The pupae produced by the flies were collected daily from larviposition cups throughout the experimental period starting from day 16-20 after emergence expected. Mating flies in cages were placed in individual larvipositioned cups and Pupae were collected from all cages daily in the morning beginning from days 16 up to the end of the experiment. All data were recorded separately per tested groups per cage and analyzed for three continuative months including their pupal size which was graded as indicated in Annex 5 [13]. The average productivity, measured as the number of Pupae Per Initial Female (PPIF), survival rate (mortality) and the number of pupae production, were measured and analyzed about tested groups. and were sorted into normal and abort larvae three by visual observation recorded [18]. Normal pupae were collected in to a separate Petri Dish, labeled according to the cage number. The pupae collected from the 20 cages of one experimental group were pooled together (90) days. Female productivity was measured as pupae per initial female (PPIF), the total number of pupae produced in a given time divided by the number of initial females. PPIF is 'commonly used to assess the health of the Glossina colonies [17].
First Larvipostion Date and Inter Larval Period: The production of pupae was recorded daily by treatment and cage. The first larval period (time between female emergence and the production of the first pupae) and the subsequent interlarval period (time between the reproductive cycles) was also recorded [17-19].
Intergenerational Effect or Pupal Quality: The pupae produced by the flies were collected daily from larviposition cups throughout the experimental period starting from day 16-20 after emergence expected and were sorted into normal and abort larvae three by visual observation recorded [18]. Abortions were recorded when females deposited under-sized larvae, at the first or second larval stage [53]. The size of pupae was measured by using a mechanical pupae sorter machine that separates the pupae based on their diameter. The sorter consists of a pair of inclined counter-rotating set to diverge by a defined size and weight. Pupae are fed on the top of the roller at the narrower end and fall into the collecting container when they have traveled along with the roller to the point when their diameter matches roller spacing. The standard system has five collecting shuts labeled A (smallest) to E (largest); the length of the collection area has been adjusted to correspond the five weight classes previously has been defined to tsetse pupae of pallidipes [13].
Statistical Analysis
Tukey multiple mean range and a one-way ANOVA with 95 confidence intervals were used to analyze the impact of the various chilling durations on reproductive efficiency for 90 days and survival rates for 120 post-exposures (response variables). Temperature and duration were then used as fixed effects and the repetitions as random effects. The temperature of 4 °C and non-chilling flies were set as reference levels (control) in all models and other treatments were compared to these values.
Post Chilling Effect on survival of pallidipes Flies over 120 days
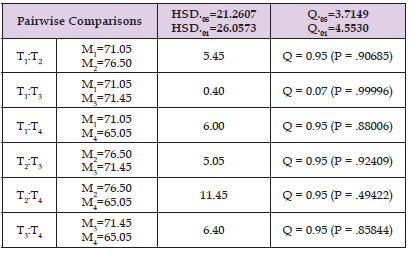
The effect of chilling on Female adult flies is shown in Table 1. In all chilling Duration, all flies were immobile but they were completely paralysed and fell to the bottom of the tubes after chilling for twenty-five minutes. All Flies in all chilling treatment appeared to recover on returning to room temperatures (23-25 °C) and no death was recorded immediately after chilling. Approximately equal numbers of the sexes were used but, to avoid complicating the table, they are not enumerated separately (Figure 1). To investigate the effect of chilling at 4oc on longevity and survival of G. pallidipes flies, a randomly selected day old teneral flies were chilled at 4 °C for 5, 10 and 25 minutes duration and compared with no chilled flies (control). Following chilling the survival of post chilled flies (a day old) were monitored for 90 days and compared with non chillied flies. The result reviled that survival of G. pallidipes flies didn't vary with chilling duration(P=0.57) (Table 1) however 76, 71.4 and 65.5 % of flies were survived after post chilling for 5, 10 and 25 minutes duration. Similarly, 70% of non chillied flies (control) was survived on 90 days) (Table 1). The minimum Lifespan in date (20) was recorded among post chilled teneral flies for 25 minutes and the maximum lifespan was recorded in non chillied flies (123 days) (Figure 1).
Table 1: Post chilling Chilling effect on survival of G. Pallidipes Tukey mean. Treatment 1, 2, 3 and 4 designated as control (non chillied), five minutes, Ten and twenty-five-minutes chilled group respectively
Post Chilling Effect on Reproductive Efficiency of Female Tsetse Flies
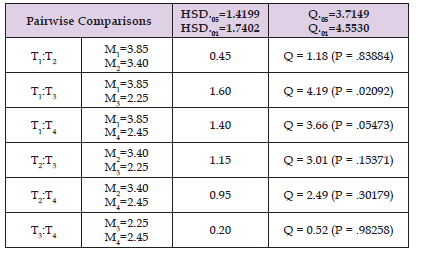
Pupae Per Initial Female of Pallidipes Flies: For consuctive 90 days Female reproductive efficiency was measured as pupae per initial female (PPIF, the total number of pupae produced in a given time divided by the number of initial females). Pupae per initial of Female G. pall G. pallidipes flies underwent chilling at 4 °C were found to be significantly lower compared with non chillied control flies. The Lowest pupae per initial female were recorded among post chilled flies for twenty-five (PPIF =2.90) and ten minutes (PPIF= 2.67) duration compared with non chillied control flies. However, there wasn't a significant difference between flies chilled for five minutes (PPIF=3.4) and non chillied flies (PPIF=3.8) (Table 2). In general the overall mean Pupae per initial of Female G.pallidipes (PPIF) was 2.98 over 90 days. Overall, among 80 experimental flies the PPIF of 12 flies (15%) were within minimum range (0-2 PPIF) and the Lowest number Non chillied flies were within the minimum range of PPIF compared with chilled flies. As shown in Figure 1 the PPIF of only 4 flies (5%) were within maximum range (6-7 PPIF) (Figures 2 & 3).
Table 2: Post chilling Chilling effect on survival of G. Pallidipes Tukey mean comparison. Treatment 1, 2, 3 and 4 designated as control (non chillied), five minutes, Ten and twenty-five-minutes chilled group respectively.
First Larvipostion Date and Inter Larval Period of Female Pallidipes
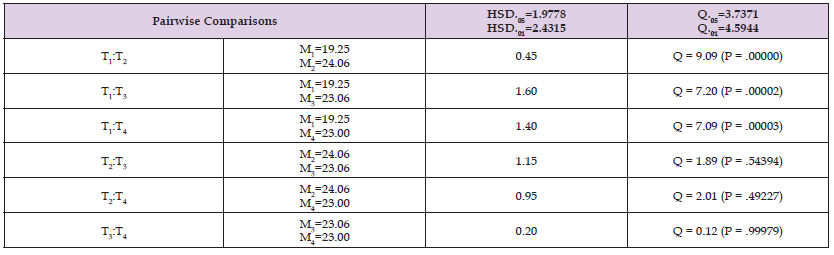
First Larvipostion Date of pallidpes: Among 16 pupae producing flies from each group , in all chilling duration, first larval deposition date was recorded and compared for 90 days. Similarly, a statically significant difference was clearly shown in their First larvipostion date among treatments (F=16.03, P value=0.001). The First larval deposition date of non chillied flies was shorter (FLPD± SD=19.25± 1.34) compared with post chilled flies at 4oc for 25 minutes (FLPD± SD=23± 2.6), ten minutes (FLPD± SD=23.06± 1.98) and Five minutes (FLPD± SD=24.06± 2.2) (Table 3).
Table 3: First larvipostion date of Female G. Pallidipes Tsetse flies in 114 days compared under different chilling duration with a one way Tukey mean comparison. Treatment 1, 2, 3 and 4 designated as control (non chillied), five minutes, Ten and twenty five minutes chilled group respectively.
Inter Larval Period: Unlike First larvipostion date (FLPD), there wasn't a significant difference between in them inter larval period (ILP) however a shorter inter larval period was recorded among non chillied flies (ILP± SD= 9.76±85) compared with post chilled flies (Table 4).
Table 4: Inter-larval period of Female G. Pallidipes Tsetse flies in 90 days compared under different chilling duration with Tukey mean comparison. Treatment 1, 2, 3 and 4 designated as control (non chillied), five minutes, Ten and twenty-five-minutes chilled group respectively.

Pupae Quality and Chilling Duration
Figure 4.
For any genetic study, male and female insects must be identified and separated. However, the labor-intensive, time-consuming, and susceptible to chilling-related productivity loss are current methods for the mechanical sorting of insects, which frequently involve chilling flies and sorting them by hand. As a result, these methods are a limiting factor in insect-based studies [16,19]. Evaluating the impact of chilling duration at 4 °C on the survival and reproductive efficiency of G. pallidpes flies was the primary goal of this experimental study. Day-old G. pallidipes flies were randomly chosen, and they were chilled at 4 °C for 5, 10, and 25 minutes. The results were compared with a control group of flies that had not been chilled. All of the flies were paralyzed and, after twenty-five minutes of chilling, they fell to the bottom of the tubes. The flies were immobile during the entire chilling duration. When the flies were brought back to room temperature (between 23 and 25 degrees Celsius), they all seemed to recover) and after chilling for more than 14 days, no deaths were reported. According to Quinn [18], the degree and duration of exposure to low temperatures can determine whether or not a chill coma is completely reversible. Yet, if the insects are exposed to extreme cold for an extended period of time, they may develop direct chilling injuries, such as membrane phase transitions, which could cause irreversible cellular damage (Quinn [18]). He also reported that Insect species were susceptible to a critical low temperature.
When exposed to a critical low temperature, many insect species would enter a coma-like state [20-25]. This comatose state is termed chill coma and is manifested in a complete arrest of movement [19]. According to Košťál et al. [21], the extent of cold injury is often assessed in terms of mortality after exposure to cold. Cold injury however, does not always result in immediate death, but sometimes cold injury can be tolerated or repaired [26]. Depending on the extent of damage and ability to repair/tolerate it, cold injury may also manifest through sub-lethal effects such as delayed mortality, delayed development, shortened lifespan and reduced fittness [24]. The results showed that, in contrast to the survival rate, the pupae per initial female of flies and the first larvipostion deposition date varied significantly with the duration of chilling (F=3.985, P value=0.0108), with the possible exception of pupae quality, which could be the result of experimental uncertainties. Cold exposure has sublethal effects, such as stunted growth, development, and decreased reproductive potential, even in the absence of mortality. In line with Colinet, et al. [25], Development can occur outside of the usual critical limits when temperatures fluctuate; however, development usually takes longer because direct cold injuries must heal. Development might even quicken if the lowest temperature does not result in harm. If there are stressful temperature fluctuations, fecundity may be decreased. In contrast to prolonged warm or cold temperatures, repeated cold exposures can raise survival and decrease supercooling points.
More inconsistent outcomes arise from repeated freeze-thaw cycles; survival is generally lower but varies by species and rises with longer recovery times and first larvipostion deposition date did significantly varying with chilling Duration (F=3.985, P value=0.0108), except pupae quality which might be due to experimental uncertainties. When mortality does not occur, exposure to cold temperatures has sublethal effects such as reduced growth, development, and reproductive potential. According to Colinet et al. [25], Fluctuating temperatures can allow development outside of the normal critical limits; however, development is typically delayed because direct cold injuries need to be repaired. If the lowest temperature does not cause injury, development may actually be accelerated. Fluctuating temperatures may reduce fecundity if stressful temperatures are experienced. Repeated cold exposures can increase survival and lower supercooling points relative to sustained warm or cold temperatures. Repeated freeze-thaw cycles have more mixed results; survival is generally decreased but depends on species and increases with increasing recovery time. For a colony to sustain- the minimum PPIF should be above 2.1 for 13 weeks [20]. However, pupae production below 3 per initial female results in no effective colony growth, or even decline [21]. Based on this fact, the results of this study indicated that both non chillied adult G. pallidipes and flies chilled a 4 °C temperature immediately after emergence resulted in the highest mean PPIF (3.85) and 3.4 respectively, in which they were found to be above the standard PPIF (PPIF=3) value required to have a steady colony growth (effective colony growth).
However, in contrast, the mean PPIF of G. pallidipes adult flies chilled at 4oc for ten (2.67) and twenty-five (2.97) minutes duration, were found to be above the standard (PPIF =2.1) value- required only to sustain or survive the colony. This result agreed with the finding of Mutika et al. [27] who found that prolonged chilling of adults affected the biological quality of the tsetse flies, hence recommending that the duration of chilling should be minimized. Additionally, handling (counting, sorting) of flies before the release process could have contributed to the overall performance of the flies because of the increased length of time under low temperatures compared to control flies. In general, the overall mean Pupae per initial of Female G. pallidipes (PPIF) was 2.98 over 90 days. Overall, among 80 experimental flies the PPIF of 12 flies (15%) were within minimum range (0-2 PPIF) and the Lowest number Non chillied flies were within the minimum range of PPIF compared with chilled flies. The PPIF of only 4 flies (5%) were within maximum range (6-7 PPIF). Willet (1933) [28] also gives results for similar work on G. austeni imported as pupae from the hot humid climate of Zanzibar and kept during the wet season under the very different climatic conditions of a grassroofed hut in the savannah. He was most impressed by the remarkable longevity and reproductivity of this species: of 18 females emerging from wild pupae, 15 had a minimum mean life of 174.5 days, and 5 exceeded 225 days; 17 of these females produced 1 6 pupae, or a mean of 6.8 pupae per female.
Foster, et al. [29], despite unsuitable maintenance climate, confirmed the promise of G. austeni; his first-generation females averaged over 7 pupae. Foster, et al. [30], has done some most valuable basic research on G. morsitans, but has confined his attention to colonies of flies kept individually in tubes, a method which so limits the output that it could not be employed for large-scale breeding. His best results were obtained from Colony 4, which started with 169 males and 177 females; the flies were kept in an incubator at 26 °C, and offered food every other day. For his first generation he achieved a mean life of 85 days for males and 90 days for females, a mean of 2.38 pupae per female and a mean inter larval period of 16.6 days; unfortunately, the investigation did not extend to further generations [31-34].
Considering all the parameters, it can be concluded that the adult flies of G. pallidipes can be immobilized and handled at 4 °C of chilling temperature for 5 minutes durations without any deterioration towards the quality of adult flies. Therefore, at any cost a subsequent chilling during handling, sex sorting and mating and releasing should be minimized and other technique should be done like Self-cage production techniques in order to avoid chilling during sex sorting and handlings.


