Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Dean Pickett1, Paulina Sarzala1, Ilan Hofmann2, Karim Fahmy2, Mahmoud Rammal2, Adel Al-Amodi2*, Amina Abane Cherrez1, Misako Kobayashi1 and Frank Merante1
Received: August 30, 2024; Published: October 03, 2024
*Corresponding author: Adel Al-Amodi, R&D Scientist, I-MED Pharma, Inc., Montreal, Quebec, Canada, H4S 2A1
DOI: 10.26717/BJSTR.2024.58.009220
This study investigates the in vitro antimicrobial efficacy of a 200 ppm hypochlorous acid (HOCl) solution (I-LID ‘N LASH HOCl CLEANSING SPRAY from I-MED Pharma Inc.), targeting microorganisms associated with ocular infections and dry eye disease. Using Colony-forming Unit (CFU) and bacteriophage plaque reduction bioassays, the study evaluates the impact of different HOCl concentrations on Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Candida albicans, Aspergillus flavus, Fusarium oxysporum and the Lambda phage virus. Results demonstrate that the 200 ppm HOCl solution achieves significant microbial reduction, with a 99.9% decrease in bacterial CFU within 10 seconds and similar reductions in fungal and viral loads. Diluted solutions (25-150 ppm) also exhibit effective microbial reduction, supporting the product’s sustained efficacy over its shelf life. Elemental and organic composition analyses confirm the product’s purity, essential for maintaining HOCl stability, effectiveness and safety. The findings highlight HOCl’s potential as a powerful antiseptic for ocular hygiene, emphasizing its role in managing dry eye disease by mitigating microbial and/or viral presence and resulting inflammation. Further in vivo studies are warranted to confirm these in vitro results.
The periocular area, which encompasses the skin and mucous membranes around the eyes, as well as the eyelid surface and margins host a diverse microbial community. This microorganism community consists of bacteria, fungi, viruses, and mites [1]. Several of these microorganisms are benign and, at times, play essential roles, provided they maintain a delicate equilibrium with the host. Disruptions to this delicate balance can result in infectious conditions related to blepharitis, conjunctivitis, keratitis, and endophthalmitis [2-4].
Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles, as defined by the Tear Film & Ocular Surface Society, Dry Eye Workshop II (TFOS-DEWS II) [5]. It has been proposed that infection could trigger inflammation of the ocular surface and instability in the tear film, resulting in symptoms of dry eye [6]. The precise relationship between dry eye and infection remains unclear; in other words, which condition precedes the other? One hypothesis suggests that dry eye increases susceptibility to ocular infection, while the other hypothesis proposes the opposite. Dry eye disease may involve a decrease in tear film quantity or quality, potentially reducing protective tear proteins. Additionally, dry eye is often linked with disruptions in the corneal epithelium, which could create opportunities for microbial invasion [7]. This theory gained support when Albeitz and Lenton (2006) found higher bacterial loads on the ocular surface of dry eye patients compared to healthy individuals [8]. However, Boiko et al.’s research suggests that ocular surface infection might predispose individuals to dry eye rather than the reverse [9]. Furthermore, Krasny et al., observed improvement in dry eye symptoms in patients successfully treated for chronic follicular conjunctivitis caused by Chlamydia infection [10].
The literature emphasizes specific microorganisms associated with various ocular infectious conditions, which in turn can contribute to disorders such as anterior blepharitis, meibomian gland dysfunction, and ocular rosacea—common culprits behind dry eye disease. At the forefront are diverse gram-positive bacteria such as Staphylococcus aureus and Staphylococcus epidermidis, gram-negative bacteria exemplified by Pseudomonas aeruginosa, fungi including Candida albicans, and filamentous fungi as represented by Aspergillus flavus, and Fusarium oxysporum, and viruses such as Measles Virus, Varicella-Zoster Virus (VZV), and Herpes Simplex Virus (HSV) [2,7,11,12].
Hypochlorous acid (HOCl) is recognized as one of the most potent antiseptic agents available, boasting antibacterial, antiviral, and antifungal properties [13]. It is naturally generated by neutrophils as part of the antimicrobial oxidative burst pathway when the body faces invasion by foreign entities [14]. Despite its remarkable effectiveness and excellent safety record, HOCl is prone to rapid degradation, presenting a challenge to its stable production for industrial or medical purposes. HOCl exhibits its highest stability within a pH range of 3 to 6. At pH levels below 3.5, the solution consists of a mixture of chlorine in the aqueous phase, chlorine gas, trichloride (Cl3−), and HOCl. As the pH exceeds 5.5, sodium hypochlorite (NaOCl) begins to form and becomes the predominant species in alkaline conditions.14 Moreover, HOCl is highly reactive and readily reacts with organic compounds containing NH2- or CHO groups, many of which are present in proteins and carbohydrates, as well as various inorganic ions like NO2-, SO3-,PO3-, Fe2+, Cu2+, and CuS. This rapid consumption of HOCl through oxidation underscores the necessity for ultra-pure ingredients to ensure the stability of the final product [15].
This study aims to evaluate the efficacy of a 200 ppm concentration of HOCl solution (I-LID ’N LASH HOCL CLEANSING SPRAY, I-MED Pharma Inc., Saint-Laurent, Quebec, Canada) in vitro by Colony-forming Unit (CFU) and bacteriophage plaque reduction bioassays, while also exploring the impact of lower concentrations of the solution. Additionally, the study examines the product’s purity concerning heavy metals and organic compounds.
Organisms
All microorganisms were purchased from ATCC (Virginia, USA) and cultured as per each species’ requirements. The microorganisms selected for this study are representative of those most commonly associated microorganisms related to ocular infectious conditions such as Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Candida albicans, Aspergillus flavus, and Fusarium oxysporum. Due to safety reasons, no human targeting viruses have been tested but instead, a surrogate non-enveloped DNA virus, bacteriophage Lambda and host E. coli cells model system have been used/ specifically, Lambda phage strain cl857 was selected, which has a mutation that prevents the virus from entering the lysogenic cycle, and the bacteria E. coli strain LE392, which is susceptible to the strain of Lambda. Therefore, the virus will not integrate as a prophage within the host, thus, directly permitting the viral concentration of the sample by assessing the phage-produced plaques. HOCl was provided by I-MED Pharma Inc. (7190 Rue Frederick Banting, Saint-Laurent, QC H4S 2A1). HOCl solution has been used within 3 months from the date of production.
Method
Bacteria and Fungi: Each microorganism was streaked on appropriate agar plates and incubated for 48 hours at 37 °C, or organismspecific temperature. On the day of the experiment, enough bacterial cells or fungal spores (conidia) were isolated and suspended in 600 μL growth media at a concentration of 5.0x108 CFU. One thousand microliters of the test solution was added to the microorganism aliquot, mixed and incubated for 10 to 60 seconds before neutralizing by dilution and before re-seeding on agar plates. Plates were incubated for 24 hours at 37 °C, or organism-specific temperature, and resulting colonies were counted.
Lambda Phage: E. coli LE392 was cultured in LB media with 0.2% maltose and 10 mM MgSO4 at 37°C. On the day of the experiment, 990 μL of LB media with 0.2% maltose and 10 mM MgSO4 was prepared into Eppendorf tubes. 50 μL of Lambda phage lysate was added to 450 μL of HOCl solution, mixed by pipetting the volume up and down, and the timer was started. At the end of each period (20 s, 40 s, and 60 s), 10 μL of the sample was transferred (HOCl solution and Lambda phage) to one of the tubes of the 990 μL of LB with 0.2% maltose and 10 mM MgSO4, and serial dilutions were performed. 100 μL of each dilution was transferred to a tube containing 300 μL of E. coli in LB media with 0.2% maltose and 10 mM MgSO4, incubated for 8 minutes at RT, then another 10 minutes in a water bath and transferred to a melted top agar tube. Sterile maltose for a final concentration of 0.2% and sterile MgSO4 for a final concentration of 10 mM was added, vortexed briefly, and quickly poured on the corresponding labelled LB plate and incubated overnight inverted at 37°C; counting was performed the next day. The experiment was performed with appropriate controls, either no E. coli or no lambda phage.
Analytical Testing: Metal analysis was conducted on I-Lid ’N Lash HOCl samples using both Thermo 6500 ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy) and Thermo Q ICP- MS (Inductively Coupled Plasma Mass Spectroscopy) which provide valuable insights into the water quality and composition that is used in the manufacturing process of HOCl. For both instruments, a semi-quantitative scan was performed, focusing on elements known for toxicity or potential harm. Each element underwent analysis with a NIST traceable standard to ensure the accuracy and reliability of results. Also, the final HOCl product was analyzed for organic acids using HP 1050 High- Performance Liquid Chromatography (HPLC) with a REZEX ROA Organic Acid Column and an HP1047A RI detector.
Staphylococcus Aureus
HOCl solution at 200 ppm (0.02%) was very effective in reducing CFU by 99.99% at 10-second exposure (4.17 log reduction). Further exposure led to more bactericidal effects but reached a plateau at 20-second exposure with a CFU reduction of 99.99% (5.25 Log reduction) till 60-second exposure. More diluted solutions, 150 ppm and 100 ppm, also lead to a reduction in CFU by 99.9% and 99.85%, after 10-second exposure (3.94 and 2.85 Log reduction), respectively. Further exposure led to an increase in bactericidal effects reaching a plateau and a comparable effect to the 200 ppm solution at 30-second exposure, killing 99.99% (5.27 Log reduction) in both solutions at 60-second exposure. The least diluted solutions, 50 ppm and 25 ppm, lead to a reduction in CFU by 99.86%, and 99.75% (2.88 and 2.62 Log reduction), after 10-second exposure, respectively. Further exposure led to an increase in bactericidal effects but did not reach a comparable effect to the 200 ppm solution, with maximum CFU reduction of 99.94% and 99.89% (3.26 and 2.97 Log reduction), respectively at 60-second exposure (Figure 1a).
Staphylococcus Epidermidis
HOCl solution at 200 ppm (0.02%) was very effective in reducing CFU by 99.96% at 10-second exposure (3.46 log reduction). Further exposure led to more bactericidal effects but reached a plateau at 40-second exposure with a CFU reduction above 99.99% (4.51 Log reduction) till 60-second exposure. More diluted solutions, 150 ppm, 100 ppm, 50 ppm and 25ppm also lead to a reduction in CFU by 99.95%, 99.68%, 99.74%, and 99.71% after 10-second exposure (3.33, 3.46, 3.19 and 3.14 Log reduction), respectively. Further exposure led to a progressive increase in bactericidal effects reaching a maximum effect of 99.98%, 99.93%, 99.96%, and 99.91% (3.87, 4.1, 4.09 and 3.82 Log reduction) reduction in CFU at 60-second exposure, respectively (Figure 1b).
Pseudomonas Aeruginosa
HOCl solution at 200 ppm (0.02%), 150 ppm and 100 ppm were very effective in reducing CFU by 99.99% at 10-second exposure (5.87, 5.87, and 5.47 log reduction). Further exposure did not lead to more bactericidal effects as a plateau was reached at 10-second exposure to 60-second exposure where CFU reduction remained at 99.99% (5.87 Log reduction for the 3 solutions). More dilute solutions of 50 ppm and 25 ppm also lead to a reduction in CFU by 99.93%, 99.90%, after 10-second exposure (3.17 and 3.02 Log reduction), respectively. Further exposure led to a progressive increase in bactericidal effects reaching a maximum effect of 99.99% and 99.97% (4.08 and 3.52 Log reduction) reduction in CFU at 60-second exposure, respectively (Figure 1c).
Candida Albicans
HOCl solution at 200 ppm (0.02%) was very effective in reducing CFU by 99.52% at 10-second exposure (2.32 log reduction). Further exposure led to more fungicidal effects reaching a maximum effect at 60-second exposure with a CFU reduction of 99.79% (2.69 Log reduction). The 150 ppm dilution also performed very well provoking a reduction in CFU of 99.46% after 10-second exposure (2.28 Logreduction). Further exposure led to an increase in fungicidal effects reaching a maximum effect at 60-second exposure, killing 99.80% (2.70 Log reduction). The least diluted solutions, 100 ppm and 50 ppm led to a reduction in CFU by 99.44%, and 99.53% (2.25 and 2.33Log reduction), after 10-second exposure, respectively. Further exposure led to an increase in bactericidal effects but did not reach a comparable effect to the 200 ppm solution, with maximum CFU reduction of 99.74% and 99.82% (2.59 and 2.76 Log reduction), respectively, at 60- second exposure (Figure 2a). Due to an oversight during the experimental process, the 25 ppm concentration was inadvertently omitted. While this omission is regrettable, the remaining conditions still provide valuable insights and contribute significantly to the overall findings of the study. Future research will aim to address this gap and include it to ensure a more complete analysis.
Aspergillus Flavus
HOCl solution at 200 ppm (0.02%) was very effective in reducing conidia CFU by 99.97% at 10-second exposure (3.59 log reduction). Further exposure led to more fungicidal effects but reached a plateau at 30-second exposure with a CFU reduction of 99.98% (greater than 3.96 Log reduction) up until 60-second exposure. The 150 ppm and 100 ppm dilutions provoked a reduction in CFU by 99.86% and 99.89% after 10-second exposure (2.86 and 3.99 Log reduction). Further exposure led to an increase in fungicidal effects reaching a comparable effect to the 200 ppm solution at 50-second exposure, killing 99,98% (greater than 3.96 and 3.82 Log reduction respectively) up until 60-second exposure. The least diluted solution, 50 ppm and 25 ppm led to a reduction in CFU by 98.73%, and 98.15% (1.90 and 1.73 Log reduction), after 10-second exposure, respectively. Further exposure led to an increase in fungicidal effects but did not reach a comparable effect to the 200 ppm solution, with maximum CFU reduction of 99.69% and 99.66% (2.51 and 2.47 Log reduction), respectively, at 60-second exposure (Figure 2b).
Fusarium Oxysporum
HOCl solution at 200 ppm (0.02%) was very effective in reducing conidia CFU by 99.77% at 10-second exposure (2.65 log reduction). Further exposure led to more fungicidal effects but reached a plateau at 20-second exposure with a CFU reduction of 99.94% (greater than 3.26 Log reduction) up until 60-second exposure. The 150 ppm, 100 ppm, and 50 ppm dilutions provoked a reduction in CFU by 99.64%, 99.40 and 99.77% after 10-second exposure (2.84, 2.23 and 2.65 Log reduction), respectively. Further exposure led to an increase in fungicidal effects reaching a plateau and a comparable effect to the 200 ppm solution at 30-second exposure, killing 99.94% (3.26 Log reduction for all dilutions) up until 60-second exposure. The least diluted solution, 25 ppm, led to a reduction in CFU of 99.51% (2.32 Log reduction), after a 10-second exposure. Further exposure led to an increase in fungicidal effects reaching a plateau and a comparable effect to the 200 ppm solution at 40-second exposure, with a maximum CFU reduction of 99.94% (3.26 Log reduction), at 60-second exposure (Figure 2c).
Lambda Phage
HOCl solution at 200 ppm (0.02%) was very effective in reducing viral load by 99.99% at 20-second exposure (4.33 log reduction). Further exposure led to more viricidal effects with reduction of virus number by 99.9991% and 99.9998% at 40 and 60-second exposure (5.03 and 5.74 Log reduction) respectively. The 150 ppm and 100 ppm dilutions provoked a reduction in virus number by 99.98%, and 99.98% after 20-second exposure (3.99 and 3.83 Log reduction), respectively. Further exposure led to an increase in viricidal effects reaching maximum effect at 60-second exposure, killing 99.99%, and 99.99% (4.83 and 4.15 Log reduction) respectively. The least diluted solutions, 50 ppm and 25 ppm, led to a reduction in virus number by 89.04% and 53.11% (0.962 and 0.33 Log reduction) after 20-second exposure, respectively. Further exposure led to an increase in viricidal effects reaching maximum effect at 60-secondexposure, killing 93.69% and 56.89% (31.20 and 0.37 Log reduction), at 60-second exposure, respectively (Figure 3).
Analytical Testing
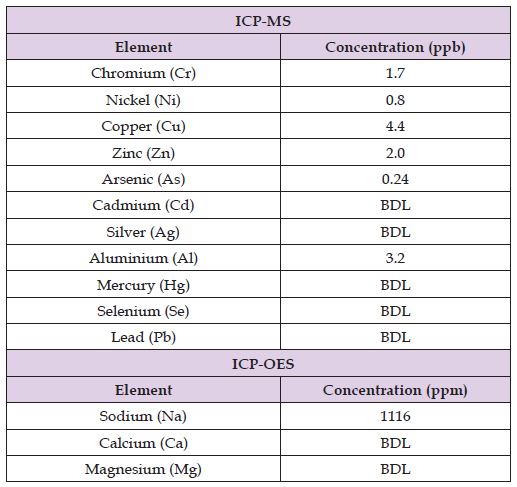
Elemental composition analysis is shown in Table 1. HPLC results didn’t confirm the presence of any organic acids, the levels were below the detection limit of 0.5 ppm.
Table 1: Elemental composition analysis of I-Lid ’N Lash HOCl CLEANSING SPRAY using Thermo Q ICP-MS and Thermo 6500 ICPOES, (ppb: parts per billion, ppm: parts per million, BDL: below detection limit, values represent the average of three replicates).
This in vitro study investigated the efficacy of HOCl solution in reducing the microorganism count. The results showed that the fullstrength product, 200 ppm or 0.02%, was capable of reducing the count of all tested bacteria by at least 99.99%, all tested fungi by at least 99.52% and the tested Lamba phase virus by 99.99% after 10 seconds exposure. Prolonged exposure, up to 60 seconds, led to an increase in the microbicidal effects. These findings align with Anagnostopoulosa et al16 previous report, it was demonstrated that 0.01% or 100 ppm HOCl solution is capable of killing 99.9% of tested bacteria and fungi within a maximum of one minute of contact, in vitro [16]. Stroman et al., also reported that 0.01% (100 ppm) HOCl solution was capable of reducing the bacterial load significantly without altering the diversity of bacterial species remaining on the skin under the lower eyelid, in vivo, by taking specimen before and 20 minutes after applying the solution and counting the CFU [17]. This underscores the significance of HOCl solution as a highly efficient tool for reducing microbial count without impacting the diversity of the ocular microbiome. Furthermore, Romanowski et al., noted that a 0.01% (100 ppm) HOCl solution effectively diminishes microbial levels without altering the diversity of biofilms. Remarkably, he highlighted HOCl as a highly potent microbicidal agent, even against antibiotic- resistant species [18]. This further underscores the value of this solution as a powerful antimicrobial for daily ocular hygiene and as a potential product for treating infectious ocular conditions.
This study investigated the effect of 200 ppm (0.02%) HOCl solution which is twice the concentration of the solution tested by Anagnostopoulosa [16], Stroman [17], and Romanowski [18]. That being said, the superiority of the 200 ppm over the 100 ppm is to be confirmed in a head-to-head study.
Various dilutions of the HOCl including 150 ppm, 100 ppm, 50 ppm, and 25 ppm, were evaluated against all microorganisms, excluding Candida albicans for the 25 ppm dilution, to assess the product’s efficacy at the end of its shelf life, confirming its persistent effectiveness. The results revealed that all tested concentrations, including the least concentrated one at 25 ppm, were capable of reducing the microorganism count by at least 99.71% for all tested bacteria, at least 98.15% for all tested fungi, and 53.11% for the tested virus after a 10-second exposure. Extended exposure of up to 60 seconds resulted in an increase of microbicidal effects. These findings validate that the product maintains its effectiveness until the very end of its shelf life, despite the anticipated significant decrease in concentration of the HOCl, potentially reaching as low as 25 ppm.
As mentioned above, this study did not assess a head-to-head comparison versus other products, especially the 0.01% HOCl product tested by Anagnostopoulos16, Stroman17, and Romanowski18 as mentioned. However, it was constantly observed that the 200 ppm full-strength product performs better than the 100 ppm dilution across all tested microorganisms. However, the real point of differentiation would be at the end of shelf- life as it is expected that the 200 ppm solution would take more time than the 100 ppm solution to drop to 25 ppm, which is the efficacy cutoff point selected by the manufacturer of I-LID ’N LASH HOCl Cleansing Spray, even though these results propose that the product maintains its efficacy even at 15 ppm. It’s also noteworthy that the experimental setup resulted in a 37.5% reduction in the measured concentration. For instance, when 1000 μL of a 200 ppm HOCl solution is mixed with a 600 μL bacterial or fungal sample, the actual HOCl concentration in the test tube is 125 ppm. Similarly, when testing the 25 ppm sample, the actual HOCl concentration in the test tube is 16.62 ppm. This approach enhances confidence in the product’s efficacy at the end of its shelf life. In the case of the lambda phage, the product was diluted by 10% making the final tested concentration, in the full strength setting, 180 ppm and not 200 ppm.
The present study was structured to assess the effectiveness of the HOCl solution against microorganisms commonly associated with ocular infectious conditions like anterior blepharitis, meibomian gland dysfunction, and ocular rosacea. HOCl solutions, ranging in concentrations from a low of 25 ppm to a high of 200 ppm, demonstrated efficacy against various bacterial and fungal species. Consequently, in vivo application of these solutions could potentially offer significant management benefits for these conditions, thus constituting a crucial component of comprehensive dry eye management protocols. For safety considerations, human viruses were not included in the testing; instead, a Lambda phage mimetic model system was developed and assessed. This specific Lambda phage, strain cl857, shares characteristics with human viruses such as the Measles Virus, Varicella-Zoster Virus (VZV), and Herpes Simplex Virus (HSV), all of which are associated with ocular infectious conditions and consequently linked to dry eye. Serrano-Aroca demonstrated that Phi 6 serves as a bio-safe viral mimetic model for SARS-CoV-2, as common disinfectants achieved comparable reductions in viral titers following similar contact durations [19] This suggests that disinfectants can be assessed using bacteriophages and the results extrapolated to human viruses. Given that the Lambda phage cl857 and the Measles Virus, Varicella-Zoster Virus (VZV), and Herpes Simplex Virus (HSV) are all non-enveloped DNA viruses, it is reasonable to extend the findings obtained with the HOCl solution using the Lambda phage cl857 to human viruses.
The broad-spectrum microbicidal impact of HOCl is very intriguing, prompting scientists to ponder over its potential mechanisms. One possible explanation lies in the formidable oxidizing power of hypochlorous acid. It effectively targets crucial cellular elements including membranes, proteins, and DNA, disrupting their normal functions [20]. This structural impairment could impede vital metabolic pathways, thereby retarding growth and cell division [21].
P. aeruginosa was demonstrated to be the most susceptible bacteria toward this treatment. The susceptibility can be attributed to specific structural characteristics of its cell wall. The Gram-negative cell wall is composed of a thin layer of peptidoglycan, located in the periplasmic space, and an outer membrane containing lipopolysaccharides and phospholipids [22]. The thin peptidoglycan layer offers less of a physical barrier to the penetration of hypochlorous acid as compared to the thicker peptidoglycan layer found in Gram-positive bacteria. Additionally, the outer membrane of Gram- negative bacteria, while acting as a barrier to many environmental stresses, is permeable to small hydrophobic molecules such as hypochlorous acid [23]. Once inside the periplasmic space, hypochlorous acid can rapidly react with various functional components of the cell, including proteins, lipids, and DNA.
The lingering question remains: how do the in vitro findings translate to in vivo applications when HOCl interacts with ions and organic substances upon skin contact? A portion of this answer is potentially addressed in the experimental design, which involved mixing 1000 μL of HOCl with 600 μL of microorganism suspension in various growth media rich in both organic and inorganic components. Additionally, Stroman et al., conducted in vivo testing by administering a 100 ppm solution onto the subjects’ facial skin, revealing a notable reduction in bioburden [17].
The analytical part of this study was performed to investigate the purity of the final product and if there are reasons to believe that impurities may jeopardize its shelf-life. As shown in Table 1, the elemental concentrations are extremely low, often falling below the detection limit. The only notable concentration is related to sodium, which arises from residual or unreacted sodium in the water due to the addition of salt (NaCl) during the manufacturing process to convert NaCl to HOCl. Using pure water in the manufacturing process of HOCl is crucial for the consistency, effectiveness, safety and stability of the final product. Pure water will ensure the chemical composition and properties of the final product, the presence of any impurities can alter the pH, conductivity, or HOCl concentration, affecting the effectiveness of the final product. Furthermore, the antimicrobial properties of the final product are optimal when it’s in pure water, the presence of impurities or contaminants in the water will reduce the efficacy of the final product against pathogens. Also, pure water minimizes the risk of having harmful substances in the final product, making it safer for the end user, especially in medical and ophthalmic applications. Finally, the stability of the HOCl can be easily affected by the quality of the water used in the manufacturing process, pure water ensures maintaining the stability and shelf-life of the final product. Additionally, organic acids, such as acetic acid, are often added to HOCl to adjust the pH level to maintain HOCl stability. However, the presence of these acids can lead to an unpleasant scent or ocular irritation when spraying the HOCl. Moreover, the presence of any organic materials can lead to a significant acceleration of the degradation of the final product. HPLC results didn’t confirm the presence of any organic acids in I-Lid ’N Lash HOCl CLEANSING SPRAY samples.
This study demonstrates that the commercially available 200 ppm HOCl solution from I-MED Pharma Inc. is highly effective in reducing the microbial load of bacteria, fungi, and a viral model in vitro, with significant reductions observed within seconds of exposure. The solution maintained its efficacy across various concentrations, indicating that it remains potent even as it approaches the end of its shelf life. The purity analysis confirmed the absence of harmful contaminants, underscoring the product’s safety for ophthalmic use, and explaining the extended shelf-life as well as the period-after- open. These results support the potential of HOCl as a powerful antimicrobial agent for managing ocular surface infections and related dry eye symptoms, highlighting its role in comprehensive dry eye management protocols.
Further in vivo studies are warranted to validate the clinical value of these findings and explore the solution’s long-term benefits in a clinical setting.
Ilan Hofmann, Karim Fahmy, Mahmoud Rammal, and Adel Al-Amodi are all employees at I-MED Pharma Inc (7190 Rue Frederick Banting, Saint-Laurent, QC H4S 2A1). The study is conducted independently at Seneca Polytechnic, Seneca Centre for Innovation in Life Sciences (70 The Pond Road, Toronto, Ontario, M3J 3M6). Elemental analysis was performed in the Department of Chemical Engineering at McGill University, Montreal, Quebec, H3A 0C5.
Research was supported by an NSERC- Innovation Enhancement Grant, and a restricted grant, from I-MED Pharma Inc.


