Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Rubens J1, Marakhouski Y Kh1*, Roshchin V2, Bartkevics V3, Rubens A1 and Zajakina A1
Received: September 04, 2024; Published: September 18, 2024
*Corresponding author: Marakhouski Y Kh, Researh and Experimtal Development and Biotechnology, BF-ESSE LLC, Brivibas Gatve 369 k.2, Riga, LV-1024, Latvia
DOI: 10.26717/BJSTR.2024.58.009192
Natural polyprenols have been studied for many years and a large number of articles have been published. However, a significant number of these publications are not indexed in official publication databases, i.e. they are classified as gray publications. Thus, over the past 5 years, only 16% of all articles on natural polyprenols have been published in peer-reviewed journals. Moreover, polyprenols themselves have complex classification characteristics that do not allow for a clear definition. It should also be noted that polyprenols are special lipids that are insoluble in water and have a low absorption threshold in their native form. To overcome this barrier, various chemical modifications of polyprenols are used, but the most physiologically complete form is the form based on fat particles in water that are close to natural micelles, which are the main ones in the physiology of lipid absorption in humans. A series of studies on the effect of natural polyprenols on immunity are characterized by fairly generalized assessments of the type of immunomodulation, immune activation with fairly vague assessments of the immune competent cells themselves and the degree of their participation in the effects of polyprenols. This primarily applies to macrophages as one of the key regulatory cells of immune reactions. The presented own research results show very promising developments in the nanoemulsion of polyprenols and their possible use for polarization of M2 macrophages, i.e. with an anti-inflammatory phenotype.
Keywords: Natural Polyprenos; Characteristics; Bioavailability; Immunity, Macrophages
Abbreviations: CNKI: Chinese Knowledge on Frastructure; CBM: Chinese Biomedical Database; RCCM: Research Council for Complementary Medicine; EMA: European Medicine Agency; FDA: Food and Drug Administration; WHO: World Health Organization; UAE: United Arab Emirates; MHC: Major Histocompatibility Complex
Plant polyprenols are a class of natural compounds with numerous descriptions of biological activity, such as anticancer, hepatoprotective and antiviral. Polyprenols anti-infective properties are particular practical importance, since their action is associated with modulation of the immune system. However, further detailing of polyprenols such effect is required, along with a systematization of existing studies of the polyprenols effects on the immune system.
The search for polyprenols publications was carried out using the main databases: PubMed, Medline, Google Scholar, ScienceDirect, Academia.edu, Web of Science, Elsevier, Researchgate.net and the main international patent databases WIPO PATENTSCOPE, ESPACENET. COM. Additional databases using: Chinese Knowledge On Frastructure (CNKI), Wan Fang, Chinese Scientific and Technological Periodical Database (VIP) and Chinese Biomedical Database (CBM), Research Council for Complementary Medicine (RCCM), British Library’s Medical Information Centre (Alternative and Allied Medicine Database), Cochrane Library Cochrane Complementary Medicine Field, Committee on Herbal Medicinal Products (HMPC) of European Medicine Agency (EMA), Food and Drug Administration (FDA), World Health Organization (WHO) Global partners commit to advance evidence- based traditional, complementary and integrative medicine.
The following keywords and their combinations (AND, OR, NOT) were used, with an emphasis on immunological effect: polyprenols, history, chemistry, analysis, classification, adverse effects, administration and dosage, agonists, inhibitors, metabolism, pharmacokinetics, pharmacology, poisoning, immunology, therapeutic use, toxicity. We selected the most important, high-quality and authoritative publications that are closely related to the problem, used the principle WordStat excels to analyze the publications text. Development option Yandex WordStat Vercion-1.9.1 (SEMANTICA). WordStat excels in text mining, providing researchers with a robust platform to delve into vast amounts of textual data. This capability enhances the depth of qualitative analysis, setting it apart in the landscape of tools for qualitative research.
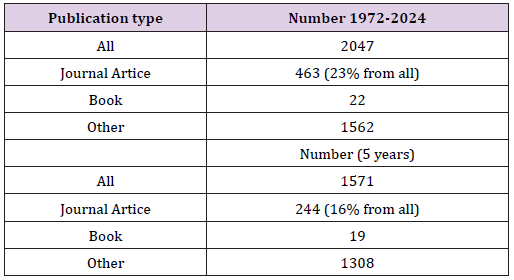
A total of 2,750 publications were found, of which 1,724 were found in the last 10 years. A significantly smaller number of publications are found in the main search databases, such as PubMed. Medical Subject Headings (MeSH) is the NLM controlled vocabulary thesaurus used for indexing articles for PubMed the term polyprenols was introduced only in 2020 (Polyprenols. Long chain isoprene compounds that include a hydroxyl group at the terminal carbon atom. Year introduced: 2020). The term immunomodulation introduced in 2010. PubMed search builder options subheadings: “Polyprenols/ immunology”[Mesh] - No results were found, “Polyprenols/metabolism”[ Mesh] - 8 results, “Polyprenols/ pharmacology”[Mesh] – 1. Therefore, the search for publications was limited to a 10-year period (2014-2024(6 months). Moreover, a significant number of polyprenols publications are not peer-reviewed journal articles. As an example, we present the search results in the database Academia.edu in Table 1. Search results show the presence of polyprenols publications are questionable in terms of the degree of evidence. Such publications are called gray. Many types of grey literature are not indexed in some of the more common research tools like PubMed, CINAHL, Scopus, Unlike Peer-Reviewed literature. Grey Literature is not reviewed by experts before being published, which means readers must do all the work of evaluating the literature themselves.
Table 1: Cross-correlations of Patient Reported Outcome questionnaire and FACIT Fatigue total and Fatigue sub-domain indices.
Other results confirming this position can be demonstrated by searching the Cochrane database. Database: Cochrane Central Register of Controlled Trials (CENTRAL), found - 12 trials matching polyprenols in Title/Abstract/Keyword. The above dictates the need to pay attention to the term polyprenols, otherwise, let’s start from the very beginning. In according to CAS Common Chemistry. CAS, a division of the American Chemical Society, n.d. plyprenols has other names for this substance: Isoprenoids, (poly-, hydroxy), Isoprenoids, Alcohols polyprenols, Polyprenol isoprenoids, Polyprenols alcohols, Alcohols, polyprenyl, Polyprenyl alcohols, Hydroxy polyisoprenoids, Polyprenyl alcs., Polyisoprenols, Ropren, Polyprenol, Polyisoprenoid alcohols. The variety of names allows us, first of all, to clearly define - what are polyprenols. The term polyprenols is not well-defined, but is generally agreed that they are natural products [1]. The significant role of polyprenols as a lipid carrier in the biosynthesis of microbial cell-surface polymers has frequently been mentioned in literature. The most important polyprenols are dolichols, ficaprenols and betulaprenols, including related compounds. These substances can be found in plants, animals and other eukaryotic organisms.
Polyprenols General Characteristics
The main sources of obtaining polyprenols are extracts from different plants and they are characterized by vary in chain lengths of their homologs and molecular configurations, as well as different numbers of isoprene components [2]. Conifer biomass such as Abies sibirica L., Picea abies L., Pinus sibirica L., Pinus sylvestris L are popular plant sources for polyprenol extraction on a commercial scale [3-5]. The highest content of polyprenols is observed in the needles of various spruce species (1.5 and 1.4% for the species Picea obovata Ledeb. and Picea abies L.. In the Abies sibirica L. extract the main polyprenols part (98%) is represented by C70H114O+C75H122O+C 80H130O+C85H138O [6]. Polyprenols, which are active ingredients identified in G. biloba, comprised of long chains of 14–24 isopentenyl units and have a similar structure as S-polyterpene alcohol (dolichols), which can be found in mammals, including people [7]. The general pathway is responsible for the production of a wide range of polyprenol structures, which vary in length across organisms, ranging from an average of C55 polyprenols in bacteria and C95 dolichols in mammals to C200 polyprenols in plants.
Bacteria typically exploit undecaprenol, a C55 unsaturated polyprenol, while archaeal organisms typically contain C55–C65 dolichols, Saccharomyces cerevisiae (yeast) contain C70–C80 dolichols and mammalian tissues contain C90–C100 dolichols [8]. The polyprenols general characteristics presented demonstrate their significant diversity and actually demonstrate the presence of several groups or families. At the same time, the structural characteristics of animals and humans differ, first of all, in isoprene components from microbes and plants. In addition, the isolation of polyprenols for research is accompanied by obtaining their mixture, and not individual molecules.
Briefly on Biological Properties
Polyprenol is used to treat Alzheimer’s disease, improves cell membrane characteristics, and has hepatoprotective, antiviral, and anti-tumoreffects. Therefore, polyprenol usage in functional foods and the development of new drugs has becomean international research hotspot [9]. Plant polyisoprenoids (PreOH and polyprenyl phosphates) are able to diminish the levels of blood cholesterol affecting its biosynthetic pathway. They also prevent toxic injuries of the liver and restore disturbed hepatic functions. At the same time polyprenyl phosphates express anti-inflammatory activity suppressing lipoxygenase activity and lowering the levels of proinflammatory cytokines [10-14]. The possibility of polyprenyl phosphates to reveal at the same time anti-inflammatory action suppressing lipoxygenase activity and lowering the levels of proinflammatory cytokines was be illustrated in several publications [15-17]. Considerable differences were found between the uptake of polyprenols of differing chain lengths. Less than 1% of the polyprenols taken up was converted into more polar product, mainly polyprenyl phosphates and polyprenyl phosphate sugars. The short-chain polyprenols, from C35 toC65, were metabolized more rapidly than the long-chain polyprenols, as judged from the amount of polar products and fatty acid esters of polyprenols [12].
These results indicate that various polyisoprenes are taken up, to a small extent, from the diet by tissues under normal conditions and in liver these dietary lipids undergo terminal modifications [18]. Worthy of mention are studies with tritium-labeled polyprenols. A search with the keywords: polyprenols and tritium and medicine revealed only 16 such studies, among them one carefully performed one should be mentioned. Asa Jakobsson, et al. [18] showed the following. Short and long dolichols and polyprenols in free form or esterified with fatty acids were incorporated into liposomes and administered to rats through a gastric tube. The free alcohols were taken up by the liver to different extents. While uptake in other organs was less, it also involved the fatty acid esters. The use of systems other than liposomes did not increase the efficiency of uptake. Most of the administered lipids were recovered in the lysosomes. Exogenous dolichols and potyprenols were both partly esterified in the liver and, to some extent, also phosphorylated; a portion of the polyprenols was also alfa-saturated. These results indicate that various polyisoprenes are taken up, to a small extent, from non-liposomes forms and in liver these lipids undergo terminal modifications.
We would like to draw attention to some data in the paper that the authors did not discuss. The authors assessed the radioactivity 20 hours after the introduction of the mixture of polyprenols into the rats stomach. Note that the established transit time in rats is the following maximal cutoff values in hours: gastric emptying -4, small intestine transit -9, and large intestine transit - 12. [19]. his means that the detected radioactivity of the labeled polyprenols is not associated with the remains of the introduced mixture in the cavity, but is associated with the polyprenols absorbed by the cells of the mucous membrane. Below is a copy of a part of the table from the authors’ work. Based on the data of the authors, we calculated the ratio in % of the share of polyprenols, the results are presented in the Tables 2 & 3. Polyprenols main share in the stomach and intestines is non-esterified and esterified dolichol. At the same time, in adipose tissue, all three polyprenols have the same share value. The results we present show the importance of dolichols for the mucous membrane.
Table 2: Copy of part of the table from the authors’ work: Uptake of various polyprenols into organs of the rat (dpm per g wet weight).

Note: FA - Fatty Acids
Table 3: The ratio of polyprenols in rat organs to the total amount according to the data in the table of the authors of the publication.

The present results show that dolichols are important for the mucous membrane cells. JACK W. RIP et al. published a high-quality systematic review of polyprenols [20], in which the following is noted in the section “B. Experiments on whole animals--intravenous injection”. As the radioactivity in plasma declined following injection of [l- 3H] dolichol, other tissues, particularly the liver and spleen, but also the intestine and contents, acquired significant amounts of tritium. About 80% of radioactivity from [1-4C]dolichol remaining in rats 24 hr post-injection was in the liver. Amounts equal to 5.9, 3.5, 3.4, 3.1 and 1.1% of the total dose were present in lung, carcass, gastrointestinal tract and contents, blood, and spleen, respectively, at this time, while each of the other organs acquired 0.1% or less of the injected radioactivity. The rate of clearance of [1-4C]dolichol from the tissues of the rat is very slow, since about half the radioactivity present in the whole rat and in the liver 24 hr after injection is still present 20 days later, almost entirely as [l-4C]dolichol. The rate of turnover in the rat carcass was even slower. In liver, esterification of dolichol to fatty acids has been observed less than 1,6%, and some phosphorylat ion of injected [1-14C] dolichol occurs. Nevertheless, more than 95% of the 4C-radioactivity in rat liver 1, 4 or 21 days after giving [1-4C] dolichol was still associated with either dolichol or its fatty acyl esters. Radiolabeled polyisoprenoid alcohols given orally to animals are poorly absorbed from the gut.
The diet, therefore, does not contribute significantly to the pool of polyisoprenoid alcohols present in animals Virus-infected cells acquired lesser amounts of [1-3H]dolichol than similar, uninfected cells. Human liver dolichol - 465 μg / g - 486 μg / g dolichol phosphate - 10.8 μg / g -11.9 μg / g [21]. Polyprenols strong hydrophobic result its low bioavailability (insoluble in water). In view of the structural characteristics of plant polyprenols containing multiple unconjugated double bonds and terminal hydroxyl groups, the research progress of polyprenols chemical modifications, such as addition, oxidation, esterification, amination, alkylation was summarized, and a series of representative polyprenols derivatives and their biological activities were introduced. Meanwhile, the latest design and development of nanoemulsions, liposomes and injections based on plant polyprenols and their derivatives were reviewed, which provide a reference for further research into the development of pharmaceutical drugs of polyprenols and their derivatives. For example: Bioeffective® R is a biologically active product extracted using Prenolica’s sophisticated patented technology from green needles of conifer species. It consists of a mixture of polyprenols (acyclic isoprenoid alcohols) homologues of very high purity (Prenolica formerly Solagran) is an Australian Biotech company, www.prenolica.com).
Bioeffective approved as an antioxidant complex, and registered and sold in Australia. It is also commercialized in the United Arab Emirates (UAE), and in Malaysia as NuvaPine® capsules. There are several significant problems with polyprenols. First of all, their high lipophilicity, they are not soluble in water. In addition, it should be noted that there are no pharmacokinetic studies of polyprenols, which is very difficult to conduct in the case of a substances with molecules mixture. In addition, studies with tritium-labeled polyprenols have shown significant differences in the polyprenols absorption and distribution in organs with different numbers of isoprene units and saturation degrees.
Effects of Polyprenols on the Immune System
The search results for the effects of polyprenols on the immune system are presented in Table 4. From Table 4 it follows. The main part of publications refers to the last 5 years - 84 (67%). In total, there are 47 publications for the analysis of the details of the polyprenols immunological effects. Below are the most important publications describing the details of immune reactions when using polyprenols. When discussing immunology (immunity), it is useful to recall the ancient Latin proverb “Repetitio est mater studiorum”, especially in the context of the correct use of terminology. Immunity, Innate: the capacity of a normal organism to remain unaffected by microorganisms and their toxins. It results from the presence of naturally occurring anti-infective agents, constitutional factors such as body temperature and immediate acting immune cells such as natural killer (MeSH PubMed definition). Innate Immunity Recognition. Innate immunity systems which recognize presence of pathogens either through detection of damages associated with pathogen effector, e.g., pathogenic toxins and pore formation (effector triggered immunity) or though detection of molecular patterns common to many types of microbes (pathogen- associated molecules, pattern molecules) through pattern recognition receptors (MeSH PubMed definition).
Table 4: Search results for the keywords “polyprenols” AND “immunity” OR “immune system” (results 125 number).

Pronin AV and co-authors [22] argue that phosphates of polyprenols may act as effective antiviral agents with a wide spectrum of activity. One of such antiviral agents received from Pinus sativum polyprenols was named phosprenyl. The drug was found to inhibit an early phase of IL-1 and Con A interaction in spleen cells as well as lypoxigenase activity and expression of IL-2receptors. At the same time, phosprenyl induced NK cell activity and early TNF-alpha production. Basing on all these data we proposed that polyprenols could be considered as a “label” which grants a possibility to the innate immune system. Some other authors express a similar point of view. For example, Safatov AS et all [23] demonstrates the possibility of achieving a prophylactic effect by intramuscular injection of Abies sibirica polyprenols for the control of influenza virus infection in mice, and concluded that emulsions of polyprenols that have relatively low hydrophilic- lipophilic balance, inhibit influenza virus infection in mice through a modulation of the host immune response. Antiviral action has been found for polyprenols obtained from other natural sources: ginkgo leaves [24], standardized extract of C. asiatica containing the triterpenoid [25] and Avicennia alba leaves [26]. The results of these studies show the presence of antiviral activity in natural polyprenols regardless of the polyprenols source. Researchers from the USA presented a randomized placebo-controlled study of the antiviral efficacy and safety of polyprenols in cats viral infection (Feline viral rhinotracheitis- FVR) [27]. Authors hypothesized that polyprenyl immunostimulant (PI), an immunomodulatory veterinary biologic, would be useful in treating feline rhinotracheitis by reducing the severity of respiratory or/and ocular disease. Authors concluded following: “Polyprenyl immunostimulant reduces clinical severity of the disease probably through immunity upregulation but has no effect on viral or antibody titers”.
Another article in this study provides more detailed results [28]. The authors have proven that comparison of mean and SD values for the survival times of cats with dry FIP showed that the survival time of cats in our study was significantly longer for the group treated with Polyprenyl Immunostimulant without concurrent corticosteroid treatment (201.4 ± 378.6 days, n = 27) than the published 38.4 ± 48.8 days (n = 11; 7); p = 0.04. In this case, leukocytosis was detected in 45% cases, lymphopenia in 33%. In this publication, the authors do not draw any conclusions about the polyprenyl immunostimulating effect. However, “Polyprenyl Immunostimulant” is listed on the Drug- Com website as a medicinal product. DrugCom: This page contains information on Polyprenyl Immunostimulant for veterinary use. Polyprenyl Immunostimulant Indications: For the treatment of cats 8 weeks of age or older against rhinotracheitis. The product license is conditional; efficacy and potency have not been fully demonstrated. Polyprenyl Immunostimulant is a 2 mg/ml solution of disodium salt of a homologous series of phosphorylated polyisoprenols with three trans- and an increasing number (7-9) of cis-isoprene isomers. Safety: Safety studies in 390 owned cats total were conducted at 24 sites in 10 states comprising 3 geographical regions. Cats’ ages ranged from 2 days to 16 years including 128 cats ≤ 8 weeks of age. 0.5 mg/kg of Polyprenyl Immunostimulant was administered to the cats orally twice daily for 15 days.
Adverse events were minimal, being confined to 3 animals reacting
to the taste of the product and one animal having diarrhea. U.S.
Veterinary License # 637. Distributed by: VetImmune, LLC, P.O. Box
205, Kingston, TN 37763. Thus, today there is already a medicinal
product in veteran medicine based on polyprenols with antiviral and
immunological activity. However, for the development of this progressive
direction, additional details of the polyprenols mechanism action
on the immune system are needed. The human microbial defense system
can be simplistically viewed as consisting of 3 levels [29]:
(1) Anatomic and physiologic barriers;
(2) Innate immunity; and
(3) Adaptive immunity.
Failure in any of these systems will greatly increase susceptibility to infection. Hematopoietic cells involved in innate immune responses include macrophages, dendritic cells, mast cells, neutrophils, eosinophils, natural killer (NK) cells, and NK T cells. Morita, et al. [30] showed that short, C5-C20 prenyl pyrophosphates may serve as antigens, the recognition of which is conserved in vertebrate evolution of γδ T cells. They offered a potential model of prenyl antigen interaction with T-cell receptors. Subset of T cells, γδT cells, exhibit specialized antigen (Ag) recognition properties and functions. γδ T cells appear to function as a bridge between the innate and adaptive immune systems and play important roles in the control of infections and autoimmune responses. To perform these functions, γδ T cells recognize unique nonpeptide Ags distinct from those recognized by ab T cells However, despite results pointing to the potential significance of those compounds in cell signaling, cellular targets for 1,4-cis-polyprenyl phosphoryl derivatives have not been identified. Let us recall. The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. TCR: two different protein chains and in humans, in 95% TCR consists of an alpha (α) chain and a beta (β) chain, whereas only in 5% of T cells the TCR consists of gamma and delta (γ/δ) chains.
It has been shown that, T cells use their TCRs to recognize self and foreign prenyl pyrophosphate intermediates (prenyl-PP) in isoprenoid synthesis Results confirm and extend recent studies and demonstrate that BTN3A1 (butyrophillin - 3BTN3) is required for the recognition of prenyl-PP but does not appear to bind the intermediates directly [31]. Detailing the polyprenols effects convincingly demonstrates their action on T-cell TRC - receptors for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. However, this process relates to a greater extent to the adaptive part of immunity and is associated with antigen- dependent differentiation of T-lymphocyte. At the same time, the first response to a viral infection is the reaction of the innate immunity. We did not find information about the state of the main components of the innate response on polyprenols in the publication databases. To produce effector and competent T cells, initially naive CD8+ T cells are activated through antigen presentation by macrophages, dendritic cells (more often), and CD4+ cells and/or directly by virus-infected cells [32,33].
To produce neutralizing antibodies, initially naive B cells are activated directly by free virus or indirectly through antigen presentation by macrophages and dendritic cells(more often), additional CD4+ cells. Naive B cells then proliferate and differentiate into plasma cells, synthesizing and secreting neutralizing antibodie, which are gradually destroyed in the serum [34]. Unlike the rapid production of effective T cells, antibody production is described by slow kinetics and is a specific characteristic of the adaptive variant of immunity. The indicated patterns of immune response to viruses have been successfully modeled in mathematical models [35]. Among the immunocompetent cells of the innate part of immunity particular importance antigen- presenting cells, which include dendritic cells and macrophages first and foremost. Moreover, dendritic cells are highly specialized in antigen presentation, while macrophages can be characterized as immune chameleons with proinflammatory (M1) or anti-inflammatory (M2) phenotypes [36]. Macrophages are a heterogeneous population of terminally differentiated cells found in all tissues, including the bone marrow. They perform essential homeostatic functions, including tissue remodeling, clearance of dead cells, and production of angiogenic factors.
The most basic function is phagocytosis of pathogens, infections, debris and dead cells. In addition, they produce different types of cytokines and join in antigen presentation by displaying processed antigens associated with major histocompatibility complex (MHC) molecules [37]. Macrophages, an important component of the innate immune response, are a key regulator of intestinal microenvironment homeostasis. These cells essentially contribute to chronic inflammatory diseases due to their strong plasticity. As is known, ulcerative colitis (UC), a chronic inflammatory disease, is closely related to immune dysfunction. A growing body of evidence suggests that the macrophage is a promising drug target for modulating the intestinal immune systems and regulating the inflammatory microenvironment, thus alleviating the inflammatory responses in ulcerative colitis. The macrophage-based therapy strategies for UC are still at an emerging stage [38].
We would like to point out the following. Two authors of this article, Victor Roshchin and Juris Rubens, has been involved in research on polyprenols for a long time and has priority in assessing the effect of polyprenols on the immune system, which is supported by two patents [39,40], with an emphasis on this option. The elaboration of immunomodulating active substance is the purpose of this invention. This purpose is achieved by using as an immunomodulating active substance from plant polyprenols with a carbon chain of fifty and more carbon atoms. Common symptoms for invention: “4. Stimulation of phagocytosis and digesting ability of macrophages”.
In our experiments, presented below, natural poliprenols was isolated from coniferous greenery, pine (Pinus Sylvestris) and spruce (Picea Abies) and carried out special purification at least 92% of the main plant-origin polyprenols homologues sum mass.
Experiment 1
The authors clearly understand the significant problem of polyprenols bioactivity and have carried out work in this direction with the consciousness of polyprenols nanoemulsion. We conducted a series of studies to create a polyprenols nanoemulsion. We managed to obtain encouraging results and obtain a stable polyprenols nanoemulsion: the droplet diameter of the dispersed phase of the polyprenols is 50-200 nm and remain stable for over 24 months at room temperature and can withstand sterilization. Patent application pending.
Experiment 2
Bone marrow-derived macrophages and their polarization. Macrophages were isolated from mouse bone marrow and cultured as previously described [41]. The procedure is schematically represented in Figure 1. Polyprenols dissolved in ethanol at 400C to achieve a polyprenols concentration 1 and 5 mg/ml. Then, this solution was diluted 1:100 with Triton-x100/BSA-containing solution: 0.1 % Triton- X100, 2 % BSA in H2O, with the final concentration of polyprenols – 10 and 50 μg/mL. The respective 0.1 % Triton-X100, 2 % BSA, and ethanol solution without polyprenol diluted 1:100 was used as a buffer control (solvent). The effect of polyprenols and the solvent on macrophage cell viability was tested using Live-or-Dye™ Fixable Viability Staining assay following flow cytometry analysis. Polyprenols optimal dose was selected with this indicator - macrophage cell viability, to rate of at least 82% viability, and final doses was 0.1 μg/ mL - 0.5 μg/mL. Preparation of bone marrow-derived macrophages (BMDM) from mouse femur and tibia. The bone marrow can be accessed from the bone ends by poking the ends with a 23G needle, slowly injecting approximately 2–3 mL PBS per bone. The cell pellet is achieved by centrifugation of the bone marrow suspension at 200 × g, 5 min at 4°C. Then the cells are resuspended in a complete medium and plated for polarization assays.
The programming of macrophages in the presence/absence of polyprenols (0.1 μg/mL and 0.5 μg/mL) was performed with mIFNg (50 ng/ml) and LPS (100 ng/ml) to achieve M1 phenotype or IL-4 (20 ng/ml) to achieve M2 phenotype, as previously described [42,43]. The macrophages were seeded into 6-well plate and the M1 or M2 polarization factors were added together with polyprenol (0.1 μg/mL and 0.5 μg/mL final concentration). Macrophages cultured with the same amount of the solvent without polyprenols and untreated cells (M0) were used as controls. The polarization of macrophages into M1 and M2 phenotypes elicits the expression of respective pro-inflammatory and anti-inflammatory markers (Figure 2). M1 macrophages are expected to produce nitric oxide (NO) through metabolic switch and upregulation of inducible NO-synthase (iNOs). The second important M1 polarization marker is the elevated expression of MHCII molecule involved in enhanced antigen presentation by M1 macrophages. On the other hand, the M2 polarization is related to CD206 upregulation, representing the main M2 polarization marker. Comments. The cell surface markers and cytokines of programmed macrophages exhibiting pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes. The M1 phenotype is induced by treatment of undifferentiated macrophages (M0) with IFNγ and LPS, or TLR1/2. The M2 phenotype is induced by treatment of M0 with IL-4, IL-13, and TGF.
In our study macrophages were cultured with IFNγ/LPS to achieve M1 phenotype in presence of 0.1 μg/mL of polyprenols. The solvent without polyprenols was used as a control or blanc. After incubation (48h), the cell medium was collected and subjected to analysis of nitric oxide, whereas the cells were stained with cell viability assay and respective antibodies followed by flow cytometry. Results presented as 2D histograms in Figure 3. As follows from the results presented in Figure 3, polyprenols do not have a polarizing effect on macrophages M0 and M1.
Experiment 3
Macrophages were cultured with IL-4 to achieve M2 phenotype in presence of 0.5 μg/mL of polyprenol. The solvent without polyprenol was used as a control. After incubation (48h), the cells were stained with cell viability assay and respective antibodies followed by flow cytometry. Results presented in Figure 4.
From the results, presented in the form of 2D histograms, is clear that polyprenols have a polarizing effect on M2 macrophages. Polyprenols at a concentration of 0.5 μg/mL exhibited stimulatory effect on M2 polarization according to anti-CD206 staining. Macrophages provide an immediate response to the penetration of a foreign pathogenic agent into the body, are one of the key cells of the innate immune system, participate in the launch and implementation of reactions of the acquired immune system. The body does not know a more reliable system for monitoring a biological parameter. Violation of macrophage functions leads to the development of chronic inflammation, autoimmune diseases, and contributes to the development and progression of oncological diseases. In the context of the above discussion, it should be noted that macrophages activation and dysregulation is also a key driver of disease progression across viral infections including SARS-CoV-2, influenza, and chikungunya viruses, underscoring the interplay between macrophages and disease progression, pathogenesis, and comorbidity in the viral infection setting [44]. The presented results of our experimental studies allow us to postulate that using polyprenols it is possible to provide immunity fine-tuning. However, further, larger-scale studies are required.


