Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Milan Perkovac*
Received: August 26, 2024; Published: September 16, 2024
*Corresponding author: Milan Perkovac, MSc, Retired Professor, University of Zagreb, Croatia
DOI: 10.26717/BJSTR.2024.58.009182
More than two and a half centuries ago Mr. Roger Joseph Boscovich proposed a type of substance as a physical quantity. But this could not be accepted, because there was no unit of measurement for the type of substance. Now, using the structure constant of all atoms, s₀ = 8.278691910, i.e. the fine structure constant α =1/(2s₀²), I proposed in his honor the unit “boscovich”, B = α = 1/137.0734794814 = 0.0072953572, for the physical quantity the type of substance. The Mendeleev’s table can be used to measure the value of that physical quantity. Because of a distinguishing number Δ in the formula of that value, its unique international tracking is necessary, for example in Sèvres near Paris. A type of substance could be called a new physical quantity, like all the other 7 basic physical quantities; m, kg, s, A, K, cd and mol, so SI (International System of Measurement Units) would thus become a system of 8 basic measurement units; m, kg, s, A, K, cd, mol, B. The new physical quantity the type of substance and its measurement unit boscovich could have a very wide application in science and in many other areas of human activity.
Keywords: Physical Quantity; Amount of Substance; Measuring Unit boscovich B; Structural Constant; Fine Structure Constant; Type of Substance
How many physical quantities exist so far? Until now, there are several dozens of basic and derived physical quantities. Of those several dozen physical quantities, according to the international SI system, only seven (7) are basic physical quantities: https://www.nist. gov/pml/owm/metric-si/si-units. All other physical quantities are derived from the aforementioned seven basic physical quantities. Every physical quantity must have its measuring unit! Without a named measurement unit, there is no physical quantity!
Examples for SI Base Units
The following table shows examples of 7 basic SI physical quantities and their measurement units and symbol: (Table 1).
Examples for derived physical quantities
Here are some examples for other (derived) physical quantities, which do not belong to the basic physical quantities: (Table 2)
Review of the Current State of Measurement Units
Since it is in the 7 aforementioned basic physical quantities, the amount of substance as a physical quantity, which gives the answer to the question of what amount of matter (substance) we are talking about, therefore, matter undoubtedly physically exists. Since matter undoubtedly exists, then it is always a question of some kind of substance, which means that for some substance there is also a physical quantity, which gives an answer to the question of what type of this undoubtedly existing substance we are talking about. Now two questions arise: how to define the value of type of substance and which measurement unit should be chosen for this physical quantity? This article deals with the answers to these two questions, i.e. how to define the value S of physical quantity type of substance and what is the unit of measurement B for that type of substance?
Whatever substance we are talking about, that substance will consist of atoms. On the other hand, atoms are composed of a nucleus, which contains protons and neutrons, and of electrons in the outer shells of atoms. If, therefore, we decompose the type of substance into its component parts, we will have protons (p is the number of protons present), neutrons (n is the number of neutrons present) and electrons (e is the number of electrons present). If we require in advance that each type of substance be unique, i.e. that two or more substances do not coincide, then we introduce the distinguishing number Δ (Δ is an integer natural number that we artificially choose to indicate the mutual difference between substances). In this way, the value of type of substance, which we denote with S, according to the Frederick Soddy, who discovered isotopes of the atoms, divided by the unit of measure B, can be written like this (Figure 1), [1], Eq. (1):
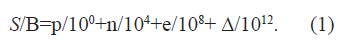
The number of protons, neutrons and electrons present for each type of substance (Mendeleev’s periodic table) can be determined from the interactive address http://periodictable.com/Isotopes/ 001.1/index.html, [2], which is shown in Figure 1.
As already mentioned, each physical quantity must have its own unit of measurement. As the unit of measurement for the type of substance cannot be derived from the existing 7 base units of measurement known so far, it follows that we have to find a new unit of measurement for the physical quantity “type of substance”. If an international agreement (convention) is reached, it is in principle possible to choose any size of the new measurement unit for the type of substance. My proposal, based on my earlier physical research, in which I calculated the structure constant of all atoms s₀ = 8.278691910, [2], is to name this unit boscovich, B, in honor of the man who before than two and a half centuries ago advocated the introduction of type of substance as physical quantity [3], and that the value of that unit should be equal to 1 B = α =1/(2s₀²), that is, that it should be equal to the numerical distance between two neighboring atoms, which total number, according to the above reference (three), can be at most 2s₀² = 137.0734795,i.e. the numerical distance between of two adjacent atoms in Mendeleev’s table is B=1/137.0734795 = 0.007295357233.
We will now show the S/B results for the above Eq. (1). S/B ratio for vacuum, electron, neutron, proton and atoms (1 to 27), S/B ratio for inorganic chemistry (28 to 45) and S/B ratio for organic chemistry (46 to 51).
In the Table 3, Z is an ordinal number in Mendeleev’s table and N is the number of atoms present.
It is evident from the previous examples that each physical quantity is represented by the corresponding Soddy number S boscovich, B or abbreviated by the ratio S/B. In this way, every existing substance or substance that will be created in the future can be described in the way shown. It is assumed that the application of the new physical quantity and its measurement unit boscovich is possible in an extremely large number of cases, starting from production, storage, the pharmaceutical industry [4] , all the way to landfills and waste sorting and processing, in the everyday field of human activity. This way of measuring and processing measurement data enables the use of bar codes and QR codes that can be used in this way with all kinds of substances, and this can be one of the biggest advantages of using a new physical quantity and its measurement unit. This can also represent a step forward for new research and development in the field of natural and social sciences. Below is a historical account of the work of Rogerio Joseph Boscovich from 1763, and the basis for the proposal to name the “boscovich”, B to the unit of measurement for the type of substance (Figure 3).
The article presents a ten-year research work on finding a new physical quantity, for which physical quantity the author proposes the name type of substance (unit sign S), and determining its unit of measurement (for which unit the author proposes the name boscovich, unit sign B), which was prompted by questions from students about how physical quantities, which indisputably exist, can be expressed from existing 7 basic measurement units of the SI system (m, kg, s, A, K, cd, mol). After it became clear that the existing SI system with its mentioned 7 basic measurement units is not able to describe, for example, smell, which is undeniably a physical quantity, it turned out that in addition to the mol, which measures the amount of substance, there is a possibility and a need to introduce a new measuring unit for type of substance. The article provides answers to two key questions, namely, how a type of substance is defined and offers a proposal for a unit of measurement for a type of substance. Measurement results for particles and atoms based on Mendeleev’s table are also presented. It is shown that any substance can be expressed using the introduced Soddy number S and the boscovich unit of measurement B, or their ratio S/B. According to the obtained results, the application of the presented solutions is possible in a very wide area of human activity. Now the international system of SI units could be expanded from the existing seven to eight basic measurement units (m, kg, s, A, K, cd, mol, B). Given that measurement units are woven into every pore of human life, all of the above can be an incentive for the development of science as a whole.
I would like to thank Ms. Sonja Fištrek Vulić, Mr. Nikola Blažević, Mr. Stjepan Trupčević-Tobi, Ms. Srebrenka Ursić, Mr. Damir Vuk, Mr. Tomislav Ivezić, Mr. Davor Pavuna, Mr. Stipe Kutleša, Ms. Ksenija Plantak, Ms. Sanja Ranty (Perkovac), Ms. Sandra Prlenda Perkovac, Ms. Nevenka Ćelepirović, Mr. Zoran Krivačić, Ms. Vesna Jarić, Mr. Srećko Jergović, Mr. Josip Zdenković, Mr. Veljko Klobučar, Ms. Ivanka Sluganović, Mr. Ivan Bahun, Ms. Barbara Zebić, Ms. Blanka Grubić, Mr. Stipe Radilović, Mr. Dražen Jakovac, Mr. Jože Muhić, Ms. Anica Bučar, Mr. Branko Počić, Ms. Slavica Lovrin, Ms. Nada Tušak, Mr. Perica Babić, Ms. Mia Perkovac, Ms. Marija (Maja) Perkovac, Mr. Zdravko Berić, Ms. Silvija Salajić, Mr. Branko Balon, Ms. Fikreta Kovačević, Ms. Ismena Meić, Ms. Mirjana Moslavac and Mr. Zlatko Voloder for their constant support and interest in my research. I would like to thank Mr. Davor Perkovac for his discusion and technical support in writing this article. I express my special gratitude to Dr. Branislav Kuzmanović, for his constant interest in my scientific work, and especially for his encouragement and emphasis on the importance of evidence when presenting my articles, as well as the introduction of discrete instead of quantum theory in my article [3], as well as the derivation of the structural constant s0 = 8.278691910, with the help of which constant the fine structure constant α=1/(2s02), von Klitzing’s constant RK = μ0cs02,Planck’s h = μ0 c e2 s02 as derived, for which it was shown that h is not a constant, and with the help of s0 were also performed the Josephson constant KJ = 2/(μ0ces02), the Rydberg constant R∞ = m/(8 μ0 e2 s06), the Bohr radius a0 = μ0 e2 s0 4/(πm), the Bohr magneton μB = μ0 c e3 s02/(4 π m) and the nuclear magneton μN = μ0 c e3 s02/(4π mP). “With the help of s0 Mr. Milan Perkovac gives a proposal for introducing eighth basic physical quantity, type of substance, with boscovich, B = α = 1/(2s0 2) = 0.0072953572 as measure unit for the type of substance, in addition to m, kg, s, A, K, cd and mol, which made an immensely large contribution to science”, says Dr. Branislav Kuzmanović. In the previous formulas, μ0 is the vacuum permeability, c is the speed of light in vacuum, e is the elementary charge, m is the mass of the electron and mP is the mass of the proton. So, the remaining 9 physical constants (α, RK, h, KJ, R∞, a0, μB, μN, and B) can be expressed using the 6 mentioned constants (s0, μ0, c, e, m, mP). Dr. Branislav Kuzmanović emphasized the mentioned facts particularly eagerly. This means that the mentioned 9 constants are actually redundant constants. B is not redundant, although it consists of s0, because B, as a number, is proposed here to be declared as the eighth basic unit of measurement for a type of substance (m, kg, s, A, K, cd, mol, B).


