Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: August 21, 2024; Published: September 12,2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant ‘Andrea 32, 56127 Pisa, Italy
DOI: 10.26717/BJSTR.2024.58.009174
Balsalazide, a prodrug of 5-aminosalicylate, is used to treat ulcerative colitis. Balsalazide is metabolized by bacteria in the colon to release its therapeutically active moiety mesalazine. In adults, the oral dose of balsalazide is 6.75 grams and balsalazide is rapidly absorbed following oral administration. The efficacy and safely of balsalazide, the treatment with balsalazide, and the trials conducted with balsalazide have been reviewed. In literature, there is only one study on the pharmacokinetics of balsalazide in humans and this study also assessed the pharmacokinetics of mesalamine. The elimination half-life of balsalazide and mesalamine is 6.1 and 7.1 hours, respectively, indicating that balsalazide and mesalamine are slowly eliminated and the toxicity induced by balsalazide has been reviewed. The aim of this study is to review the efficacy and safely of balsalazide, the treatment with balsalazide, and the trials conducted with balsalazide. In addition, the pharmacokinetics of balsalazide and the toxicity caused by balsalazide have been reviewed.
Keywords: Balsalazide; Efficacy-Safely; Pharmacokinetics; Toxicity; Treatment; Trials
Balsalazide is a prodrug of 5-aminosalicylate which is used to treat ulcerative colitis. Balsalazide is metabolized by bacteria in the colon to release its therapeutically active moiety mesalazine. In adult, the oral daily dose of balsalazide is 6.75 grams and balsalazide is rapidly absorbed following oral administration. The absorption of balsalazide is partly absorbed from the stomach but mostly from the upper small intestine. The rate of absorption is determined by disintegration and dissolution-rates of the tablet administrated, by the pH of the mucosa surface, and by gastric emptying time and food delays the absorption of balsalazide. After absorption, balsalazide is distributed throughout most body tissues and transcellular fluids [1] (Figure 1).
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “balsalazide efficacy, safely”, “balsalazide treatment”, “balsalazide trials”, “balsalazide pharmacokinetics”, and “balsalazide toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Seven studies have been reported on the efficacy and safely of balsalazide. Balsalazide, administered orally at the daily dose of 6.75 grams to 21 patients with inflammatory bowel disease, was well-tolerated and effectively and safely treated the patients [2]. Long-term treatment (up to 1.4 years) with balsalazide, administered orally at the daily dose of 3.3 grams, effectively and safely treated patients with ulcerative colitis [3]. Balsalazide, administered orally at the daily dose of 6.75 grams, was safe, well-tolerated, and was more efficacious (P-value < 0.05) than mesalamine, administered orally at the daily dose of 2.4 grams, for treatment of patients with ulcerative colitis [4]. Balsalazide was administered orally at the daily dose of 6.75 grams and mesalamine was administered orally at the daily dose of 2.4 gram to patients with ulcerative colitis and balsalazide was more efficacious (P-value < 0.05) and better tolerated than mesalamine in treatment of patents [5]. Balsalazide was administered orally at the daily dose of 6.75 mg to patients with mild-to-moderate ulcerative colitis and balsalazide effectively and safely treated the patients [6]. Balsalazide was administered orally at the daily dose of 6.75 grams, mesalazine was administered orally at the daily dose of 2.4 grams, and sulfasalazine was administered orally at the daily dose of 3 grams to patients with ulcerative colitis. Balsalazide was more efficacious, safer, and better tolerated than mesalazine and sulfasalazine [7]. Balsalazide was administered orally at the daily dose of 6.75 grams and mesalazine was administered orally at the daily dose of 2.4 grams to patents with ulcerative colitis. Balsalazide was well-tolerated and was more efficacious (P-value < 0.05) than mesalazine in treatment of patients [8].
Ten studies have been reported on the treatment with balsalazide. Balsalazide was administered orally at the low-dose of 1.5 grams twice-daily, balsalazide was administered at the high-dose of 3 grams twice-daily, and mesalazine was administered orally at the dose of 0.5 grams thrice-daily to patients with ulcerative colitis. High-dose balsalazide was superior to low-dose of balsalazide and to mesalazine in treatment of patients and all treatments were well-tolerated [9]. Balsalazide was administered orally at the daily dose of 6.75 grams and mesalamine administered orally at the daily dose of 2.4 grams to patients with ulcerative colitis. Balsalazide was an effective and safe treatment of ulcerative colitis and was more efficacious and safer than mesalamine [10]. Balsalazide was administered orally at the daily dose of 6.75 grams and sulfasalazine was administered orally at the daily dose of 3 grams to patients with ulcerative colitis and balsalazide was more efficacious (P-value < 0.05) than sulfasalazine in treatment of patients and balsalazide was better tolerated [11]. Balsalazide was administered orally at the daily dose of 6.75 grams and mesalazine was administered orally at the daily dose of 3 grams to patients with ulcerative colitis. Balsalazide was more efficacious (P-value < 0.05) than mesalazine and both drugs were well-tolerated [12]. Balsalazide, administered orally at the daily dose of 6.75 grams, effectively treated patients with mild-to-moderate ulcerative colitis and prevented the recurrence of uncomplicated diverticulitis of the colon [13].
It was compared the efficacy and the tolerability of sulfasalazine to those of mesalamine, olsalazine, and balsalazide in patients with ulcerative colitis. Sulfasalazine did not differ from mesalamine, olsalazine or balsalazide in terms of efficacy and tolerability in patients with ulcerative colitis. Sulfasalazine, mesalamine, olsalazine, and balsalazide were similarly efficacious in treatment of patients with ulcerative colitis and were well-tolerated [14]. Balsalazide, administered orally at the daily dose of 6.75 grams, effectively treated patients with ulcerative colitis [15]. Balsalazide was administered orally at the daily dose of 3 grams for three years to patients with ulcerative colitis. Long-term treatment with balsalazide was well-tolerated with a good safety profile and balsalazide effectively treated the patients [16]. Balsalazide, mesalazine, and sulfasalazine effectively treat ulcerative colitis. When balsalazide was compared to mesalazine balsalazide caused complete remission of acute ulcerative colitis in a greater proportion of patients. Balsalazide had a similar efficacy in reducing symptoms of ulcerative colitis as sulfasalazine and mesalazine [17]. Balsalazide was administered orally at the daily dose of 6.75 grams or at a daily dose of 2.25 grams and mesalamine was administered orally at the daily dose of 2.4 grams to patients with ulcerative colitis and the treatments lasted 8 weeks. Treatment with high-dose of balsalazide was significantly more efficacious (P-value < 0.05) than treatment with low-dose of balsalazide and balsalazide more rapidly improved signs and symptoms of acute ulcerative colitis [18].
Two trials conducted with balsalazide have been reviewed. A multicenter, randomized, active-control, double-blind, double-dummy, dose-response, parallel-group trial compared balsalazide administered orally at the daily dose of 6.75 grams to balsalazide administered orally at the daily dose of 2.25 grams and to mesalamine administered orally at the daily dose of 2.4 grams in patients with mild-to-moderate ulcerative colitis and treatments lasted 8 weeks. Treatment with 6.75 grams of balsalazide was more efficacious (P-value < 0.05) than treatment with balsalazide administered at the dose of 2.25 grams and mesalamine was more rapidly (P-value < 0.05) absorbed than balsalazide. Balsalazide and mesalamine were well-tolerated and treated patients with mild-to-moderate ulcerative colitis [19]. A multicentre, double-blind trial was conducted in 68 children, aged 5 to 17 years, with mild-to-moderate ulcerative colitis who received balsalazide orally at a daily dose of 2.25 or 6.75 grams for 8 weeks. Clinical improvement was achieved by 45% and by 37% children and clinical remission was achieved by 12% and by 9% children who received balsalazide at the daily dose of 6.75 and 2.25 grams, respectively. Balsalazide was well-tolerated and improved signs and symptoms of mild-to-moderate active ulcerative colitis in children [20].
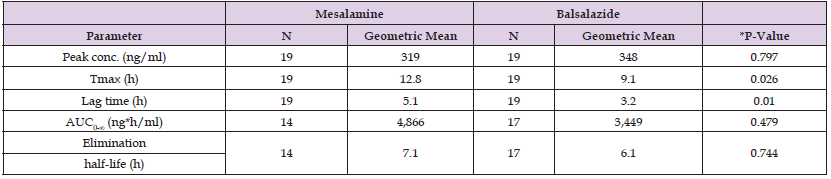
In literature, there is only one study on the pharmacokinetics of balsalazide in humans and it has been reported by Sandborn, et al. [21]. These authors studied the pharmacokinetics of balsalazide and mesalamine in 19 healthy volunteers. A single oral dose of 800 mg of balsalazide or a single oral dose of 2,250 mg of mesalamine was administered to volunteers. Table 1 provides the vital data of volunteers included in the study and Table 2 summarizes the pharmacokinetic parameters of mesalamine and balsalazide. This table shows that mesalamine and balsalazide are slowly eliminated as the elimination half-life is 7.1 and 6.1 hours, respectively. Mesalamine is absorbed more slowly than balsalazide as the time to reach the peak concentration is 12.8 hours for mesalamine and 9.1 hours for balsalazide. The lag of time is 5.1 hours for mesalamine and 3.2 hours for balsalazide. The other pharmacokinetic parameters obtained with mesalamine are not significantly different from those obtained with balsalazide.
Table 1: Vital Data of Volunteers included in the Study. Values are the mean+SD or the mean and (percentage), by Sandborn, et al. [21]).
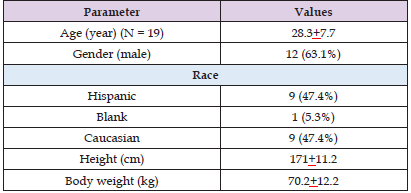
Table 2: Pharmacokinetic parameters of mesalamine and balsalazide which have been obtained in 19 healthy volunteers who received a single oral dose of 800 mg of balsalazide or a single oral dose of 2,250 mg of mesalamine. Values are the geometric mean and the number of volunteers included in the study, by Sandborn, et al. [21].
Note:
Tmax = time to reach the peak concentration. Lag time = the lag of time before the onset of drug absorption. AUC = area under the concertation-time curve. *ANOVA.
Four studies have been conducted on the toxicity of balsalazide. A 68-year-old woman with ulcerative colitis was treated with balsalazide and presented several months of chest pain, orthopnoea, and dyspnoea [22]. A woman, treated with balsalazide for ulcerative colitis, had eosinophilic pneumonia and presented cough, progressive dyspnoea, and lethargy. Her chest radiograph showed left-sided infiltration, high-resolution computerized tomography demonstrated widespread nodular shadowing and opacification in the left lung, and trans-bronchial lung biopsy confirmed eosinophilic pneumonia [23]. Balsalazide is associated with rare but potentially life-threatening adverse-effects such as pericarditis, myocarditis and pneumonitis. These adverse-effects appear to be caused by a hypersensitive reaction to balsalazide and resolve after cessation of balsalazide [24]. A patient with ulcerative colitis was treated with balsalazide and presented respiratory symptoms and multiple pulmonary nodules. The chest radiography showed a limited form of granulomatosis with polyangiitis and with bronchiolitis [25].
Balsalazide is a prodrug of 5-aminosalicylate which is used to treat patients with ulcerative colitis. Balsalazide is metabolized by bacteria in the colon to release its therapeutically active moiety mesalazine. In adults, the oral daily dose of balsalazide is 6.75 grams and balsalazide is rapidly absorbed following oral administration [1]. The efficacy and safely of balsalazide have been reviewed. Balsalazide, administered orally at the daily dose of 6.75 grams to patients with inflammatory bowel disease, is well-tolerated and effectively and safely treats the patients [2], long-term treatment, up to 1.4 years, with balsalazide administered orally at the daily dose of 3.3 grams effectively and safely treats patients with ulcerative colitis [3], balsalazide, administered orally at the daily dose of 6.75 grams, is safe and well-tolerated and balsalazide is more efficacious (P-value < 0.05) than mesalamine which was administered orally at the daily dose of 2.4 grams for treatment of patients with ulcerative colitis [4], balsalazide was administered orally at the daily dose of 6.75 grams and mesalamine was administered orally at the daily dose of 2.4 grams to patients with ulcerative colitis. Balsalazide is more efficacious (P-value < 0.05) and better tolerated than mesalamine in treatment of patients [5], balsalazide, administered orally at the daily dose of 6.75 grams, effectively and safely treats patients with mild-to-moderate ulcerative colitis [6], balsalazide was administered orally at the daily dose of 6.75 grams, mesalazine was administered orally at the daily dose of 2.4 grams, and sulfasalazine was administered orally at the daily dose of 3 grams to patients with ulcerative colitis.
Balsalazide is more efficacious, safer, and better tolerated than mesalazine and sulfasalazine [7], and balsalazide was administered orally at the daily dose of 6.75 grams and mesalazine was administered orally at the daily dose of 2.4 grams to patients with ulcerative colitis and balsalazide is well-tolerated and is more efficacious (P-value < 0.05) than mesalazine in treatment of patients [8]. The treatment with balsalazide has been reviewed. Balsalazide was administered orally at the low-dose of 1.5 grams twice-daily, and was administered at the high-dose of 3 grams twice-daily, and mesalazine was administered orally at the dose of 0.5 grams thrice-daily to patients with ulcerative colitis. High-dose of balsalazide is superior to the low-dose of balsalazide and to mesalazine in treatment of patients and all treatments are well-tolerated [9], balsalazide was administered orally at the daily dose of 6.75 grams and mesalamine was administered orally at the daily dose of 2.4 grams to patients with ulcerative colitis. Balsalazide effectively treats the patients and is more efficacious and safer than mesalamine [10], balsalazide was administered orally at the daily dose of 6.75 grams and sulfasalazine was administered orally at the daily dose of 3 grams to patients with ulcerative colitis. Balsalazide is more efficacious (P-value < 0.05) than sulfasalazine and balsalazide is better tolerated [11], balsalazide was administered orally at the daily dose of 6.75 grams and mesalazine was administered orally at the daily dose of 3 grams to patients with ulcerative colitis and balsalazide is more efficacious (P-value < 0.05) than mesalazine and both treatments are well-tolerated [12].
Balsalazide, administered orally at the daily dose of 6.75 grams, effectively treats the patients with ulcerative colitis and prevents the recurrence of uncomplicated diverticulitis of the colon [13], sulfasalazine, mesalamine, olsalazine, and balsalazide are similar efficacy in treatment of patients with ulcerative colitis and are well-tolerated [14], balsalazide, administered orally at the daily dose of 6.75 grams, effectively treats patients with ulcerative colitis [15], balsalazide was administered orally at the daily dose of 3 grams for three years to patients with ulcerative colitis and long-treatment with balsalazide is safe and treats the patients [16], balsalazide, mesalazine, and sulfasalazine effectively treats patients with ulcerative colitis. When balsalazide is compared to mesalazine it causes complete remission of acute ulcerative colitis in a greater proportion of patients. Balsalazide has a similar efficacy in reducing symptoms of ulcerative colitis as sulfasalazine and mesalazine [17], and balsalazide was administered orally at the daily dose of 6.75 grams or at the daily dose of 2.25 grams and mesalamine was administered orally at the daily dose of 2.4 grams to patients with ulcerative colitis and treatments lasted 8 weeks. Treatment with high-dose of balsalazide is more efficacious (P-value < 0.05) than treatment with low-dose balsalazide and balsalazide more rapidly improves signs and symptoms of ulcerative colitis [18]. The trials conducted with balsalazide have been reviewed. A multicentre, randomized, active-control, double-blind, double-dummy, dose-response, parallel-group trial compared balsalazide administered orally at the daily dose of 6.75 grams to balsalazide administered orally at the daily dose of 2.25 grams and to mesalamine administered orally at the daily dose of 2.4 grams in treatment of patients with mild-to-moderate ulcerative colitis and treatments lasted 8 weeks.
High-dose of balsalazide is more efficacious (P-value < 0.05) than the low-dose of balsalazide in treatment of patients and mesalamine was more rapidly absorbed (P-value < 0.05) than balsalazide [19], a multicenter double-blind trial was conducted in children with mild-to-moderate ulcerative colitis. Children received balsalazide orally at the daily dose of 2.25 or 6.75 grams for 8 weeks. Clinical improvement is achieved by 45% and by 37% and the clinical remission was achieved by 12% and by 9% children who received the high-dose and the low-dose, respectively, of balsalazide. Balsalazide is well-tolerated and improves the signs and symptoms of ulcerative colitis [20]. In literature, there is only one study on the pharmacokinetics of balsalazide in humans and it has been reported by Sandborn, et al. [21]). These authors studied the pharmacokinetics of balsalazide and mesalamine in healthy volunteers. A single oral dose of balsalazide of 800 mg and a single oral dose of mesalamine of 2,250 mg were administered to 19 healthy volunteers. The elimination half-life of balsalazide and mesalamine is 6.1 and 7.1 hours (P-value = 0.744), respectively, suggesting that both balsalazide and mesalamine are slowly eliminated. The time to reach the peak concentration of balsalazide and mesalamine is 9.1 and 12.8 hours (P-value = 0.026), respectively, indicating that balsalazide is more rapidly absorbed than mesalamine. The toxicity induced by balsalazide has been reviewed. A 68-year-old woman treated with balsalazide for ulcerative colitis presented several months of chest pain, orthopnoea, and dyspnoea [22], a woman, treated with balsalazide for ulcerative colitis, had eosinophilic pneumonia and presented cough, progressive dyspnoea, and lethargy.
Her chest radiography showed left-sided infiltration, high-resolution computerized tomography demonstrated widespread nodular shadowing and opacification in the left lung, and trans-bronchial lung biopsy confirmed eosinophilic pneumonia [23], balsalazide is associated with rare but potentially life-threatening adverse-effects such as pericarditis, myocarditis, and pneumonitis. These adverse-effects appear to be caused by a hypersensitive reaction to balsalazide and resolve after cessation of balsalazide [24], and a patient treated with balsalazide for ulcerative colitis presented respiratory symptoms and multiple pulmonary nodules and the radiography sowed a limited form of granulomatosis with polyangiitis and with bronchitis [25]. These studies suggest that balsalazide may induce toxicity. In conclusion, balsalazide is a prodrug of 5-aminosalicylic which is used to treat ulcerative colitis. Balsalazide is metabolized by bacteria in the colon to release its therapeutically active moiety mesalazine. In adults, the oral dose of balsalazide is 6.75 grams. The efficacy and safely of balsalazide, the treatment with balsalazide, and the trials conducted with balsalazide have been reviewed. In literature, there is only one study on the pharmacokinetics of balsalazide in humans and the elimination half-life of balsalazide is 6.1 hours. This study also assessed the pharmacokinetics of mesalamine and the elimination half-life of mesalamine is 7.1 hours, suggesting that balsalazide and mesalamine are slowly eliminated. The toxicity induced by balsalazide has been reviewed and balsalazide causes different toxicities. The aim of this study is to review the clinical pharmacology of balsalazide.
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals.
The author conceived, wrote, and typed the present manuscript. Prof. Gian Maria Pacifici, via Sant ‘Andrea 32, 56127 Pisa, Italy, is the corresponding author. The author is responsible for the reported research. He conceived and designed the study, executed the analysis, interpreted the results, and he drafted, revised, and approved the manuscript as submitted. The present article is a review and drugs have not been administered to men or animals.
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.


