Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Umra Rasool1*, Ayeza Tabassum2, Tahira Pechuho3, Wajeeha Rafaqat4, Fatima Noreen5 and Syeda Maheen Abid6
Received: August 22, 2024; Published: September 04,2024
*Corresponding author: Umra Rasool, Department of Veterinary Sciences, University of Torino, Italy and Government College Women University Faisalabad
DOI: 10.26717/BJSTR.2024.58.009161
Substituting plant protein with fish meal is favorable because it is economical, environmentally safe and can be reciprocal to meet the nutritional needs of fish. The current research was conducted to evaluate the effect of 30% and 50% plant protein mixture along with probiotics addition on the growth production and intestinal morphology of Labeo rohita. This research was conducted at the Fish Nutrition Laboratory, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad. Ten fingerlings of L. rohita were kept in each of the five glass aquaria making groups T0, T1, T2, T3 and T4 with triplicates for a period of four months. L. rohita fingerlings were given 35% crude protein @ 6% of body weight. Group T0 was control group and fingerlings were fed with 100% fish meal, while T1, T2, T3 and T4 were experimental groups in which T1 was given 30% plant protein mixture with 2% probiotics, T2 was given 30% plant protein mixture with 4% probiotics, T3 was given 50% plant protein mixture with 2% probiotics and T4 was given 50% plant protein mixture with 4% probiotics. At the end of the trial, samples of the fish intestine from experimental group and control group were analyzed to calculate intestinal morphology in terms of villus height (µm) and villus width (µm). The villus height (µm) and villus width (µm) of L. rohita showed significant results in T2. Data was analyzed statistically by using ANOVA under completely randomized design (CRD). Growth performance in terms of body weight (g), body length (cm), feed conversion ratio (FCR) and specific growth rate (SGR) were measured on weekly basis. Maximum weight gain was recorded in experimental group T2 (102±0.14). Maximum value of length gain was recorded in T2 (21.9±0.15). Highest value of SGR was recorded in T2 (2.07±0.31). Physico-chemical parameters were measured after every week. The results of this experiment suggested that it is possible to replace 30% and 50% of fish meal with plant protein mixture in the feed of L. rohita without negatively affecting the growth production and intestinal morphology.
Keywords: Aquaculture; Breeding; Decorative Species; Fish Production
Abbreviations: SFM: Sunflower Meal; ANFS: Anti-Nutritional Elements; FCR: Feed Conversion Ratio; SGR: Specific Growth Rate; EBT: Eriochrome Black T; EDTA: Ethylene Diamine Tetra Acetic Acid; PPM: Parts Per Million; AA: Amino Acid
Aquaculture is the activity of breeding, keeping, and raising of fish and other aquatic animals like carps and shrimps, plants like seaweeds and algae under full or partial human control. Aquaculture in this way maintain the biodiversity of the environment and provide recreational opportunities for people (Galappaththi, et al. [1]). Aquaculture has become the fastest growing food producing sector in the world. The need for aquatic food products is rising as the global population is increasing. Aquaculture continues to dominate aquatic food production in Asia and globally. Aquaculture has great impact on world’s economy (Yue, et al. [2]). The consumption of fish and seafood occupies a significant place in the world's supply of food to the population (FAO, et al. [3]). Aquaculture has been primarily used to create food since its inception, which dates back at least 4,000 years ago. It has also been used to produce other commercial goods, including as pharmaceuticals, decorative species for aquariums and substrate for commercial applications (Mizuta, et al. [4]). Over the past 50 years, the number of fish consumed globally per person has almost doubled. Over the past 25 years, the growth in aquaculture output has outpaced that of the majority of other food commodities. By 2020, 56% of fish that were directly available for human consumption worldwide came from aquaculture (Naylor, et al. [5]). Aquaculture provides 47% of entire worldwide fish production and it will increase its share about 52% in 2025 (FAO, et al. [3]).
Fish are the most diverse aquatic vertebrates and can be found in practically all aquatic environments. Fish is a great source of essential nutrients for human health, such as vitamins, minerals, omega-3 fatty acids and proteins. Over a billion people are estimated to rely on the production and trade of fish for their existence on a global basis (Idowu, et al. [6]). The total estimated number of fish produced globally by aquaculture and capture fisheries was 178.5 million tons in 2018 (Thirukumaran, et al. [7]). Fish is a nutrient-dense food that makes up around 17% of the world's animal protein intake and a cheap source of protein for humans (Maschmeyer, et al. [8]). The need for fish protein components is gradually increasing on a global scale. Fish proteins can be divided into Sarcoplasmic, stroma and myofibrillar proteins (Xu, et al. [9]). Freshwater aquaculture used to rear many carps, most important of them is Indian major carp. Indian major carp known as Labeo rohita is the most cultivable fish species in carp polyculture due its rapid growth, public demand, high nutritional content and delicious flavor (Maryam, et al. [10]). L. rohita is an important freshwater fish and usually cultured in Asian region. Production of L. rohita seed in captivity motivate farmers to increase the amount of this species due to establishment of successful hatchery technology (Choudhary, et al. [11]). Farm bred L. rohita require diet that has 30–35% protein because they are omnivores. Its fecundity is two lac eggs per kg (Rasal, et al. [12]).
The rapid growth of worldwide aquaculture production raises demand for aqua feeds. Feeds provide nutrients to fish, allowing them to grow faster, survive longer, and live healthier lives (Hodar, et al. [13]). The fish farmers have no access to any useful feed so many mesh feeds are being utilized to increase fish productivity (Husain, et al. [14]). The aquaculture industry is currently faced with challenges to meet food demand due to increase in population. Fishmeal is considered a premium protein source in diets for aquaculture fish (Monteiro, et al. [15]). Fish meal is the raw flour obtained from the drying and grinding of fish meal. Fish meal improves nutritional uptake, digestion and absorption while also increasing feed efficiency, quality and faster growth through improved food palatability (Hodar, et al. [13]). Fish meal is the main protein source in aquaculture feeds due to its high digestibility and palatability and it has balanced amino acid composition (Riche, et al. [16]). In terms of cost, plant protein components such as soybean, canola seeds, cottonseed meal and sunflower meal have gained the most support among all alternative sources because they look to be less costly than fishmeal (Soltan, et al. [17]). The developing and less expensive protein sources can replace fish meal without compromising the performance of the animal would be crucial for the development of aquaculture in future (Yao, et al. [18]). Plant protein mixture have been employed to reduce the use of fish meal. Plant feeds such as soybean meal have long been the preferred choice due to their high nutritional value, low cost, and consistent availability (Msonteiro, et al. [15]).
Plant sourced feed stuffs used in aquaculture include soybean meal, fermented soybean meal, leaf meal, wheat, wheat hydrolyzed protein, cotton seed meal, canola meal, peanut meal and sunflower meal (Jia, et al. [19]). Majority of different protein sources are derived from plants to replace fish meal. The plant protein sources, such as rapeseed, cottonseed, corn gluten meals, plant by-products and other plant meals are used successfully in the feed of various fish species to partially replace fish meal. The most important features of these alternate protein sources are that they have similar range of essential amino acids, phospholipids and fatty acids (Daniel, 2018). These sources are also easily available, abundant and cheap (Ayadi et al., 2012). It has 40–60% crude protein content and is rich in minerals and functional factors (iso-flavones) (Thangaviji, et al. [20]). Soybean meal is considered as an appropriate protein source and good alternative for fish meal in aqua-feed because of its nutritional composition, easy availability, affordable price and beneficial amino acid profile (Krishnan, et al. [21]). Sunflower meal presents a viable substitute for fish meal as a source of protein due to its reduced cost. Sunflower meal (SFM) has a crude protein value of 36–40%. Additionally, it contains high levels of pantothenic acid, pyridoxine, biotin, choline, niacin, riboflavin, and vitamin E. Sunflower meal contain high quantities of selenium, iron, zinc, magnesium and potassium (Iqbal et al., 2022). Cotton seed is the second-largest protein source used in animal feed.
It is a valuable component of animal feed component due to its relatively high protein material and easy availability (Dharmakar, et al. [22]). Cottonseed meal has protein content up to 45 to 55% with balanced amino acids. Animal feed additives are used throughout the world to maximize feed utilization, boost palatability, improve growth performance, and supply vital nutrients to animals. Growth performance must maintain a high level of health, and in these situations, using the right additives is a major point of contention. Probiotics, prebiotics, enzymes, and herbs are a few substitutes that are being considered for use as additives in animal feed (Pandey, et al. [23]). Feed additives are non-nutritive ingredients or non-nutritive parts of ingredients that are added to formulations to change the chemical or physical characteristics of the food, the performance of aquatic animals, or the caliber of the products that are produced. Other additives such as probiotics, prebiotics, acidifiers, and extracts derived from plants or animals have a direct impact on fish performance or product quality (Dawood, et al. [24]). Herbs improved fish growth and feed efficiency, while also reducing diseases by regulating pathogens in the gastrointestinal tract (Ogunkalu, et al. [25]). More study is needed on novel feed additives, such as using herbs in fish meals to cut costs and improve digestibility. Probiotics in aquaculture has been acknowledged to improve growth, immunity and utilization of nutrients by lowering the manufacturing expenses (Safari, et al. [14]).
Probiotic supplementation in feed may be a nutritional method that improves aquaculture production through appropriate nutrient use while also lowering production costs, ensuring the sector's long-term sustainability and profitability (Jahan, et al. [26]). Regarding these issues, the feed additives particularly probiotics are used as an alternative strategy in aquaculture for disease control (Hoseinifar, et al. [27]). Adding probiotics into the feed can improve the growth and development of the gut and overall health of species used in aquaculture (Ramos, et al. [28]). Supplementation of diet with bacteria as probiotics showed maximum dietary performance and growth of fingerlings of L. rohita. In conclusion, the replacement of plant protein mixture with fish meal has positive impact on the growth performance of L. rohita due to its protein content. Feed additives are used to enhance the digestibility of plant protein. The application of probiotics with plant protein can improve growth performance of L. rohita by inhibition of the growth of pathogens, alteration of gut and stimulation of immune responses (Mohammadian, et al. [29]). Probiotics with plant protein improve feed digestion and nutrient absorption in the host stomach. Probiotics are now widely recognized as a promising environmentally friendly and biological agent for preventing infectious diseases in farmed aquatic animals by altering the host's gut microbiome (Salehi, et al. [30]). The objectives of present research are to assess the growth production of Labeo rohita fed with 30% and 50% plant protein mixture. And to evaluate the intestinal morphology of L. rohita under the effect of 30% and 50% plant protein and probiotics.
This research was conducted to evaluate the effect of 30% and 50% plant protein mixture (cotton seed meal, soybean meal and sunflower meal) and probiotics on growth and intestinal morphology of Labeo rohita. L. rohita fingerlings were collected from the Fisheries Research Farm, University of Agriculture, Faisalabad. This experiment was conducted at the Fish Nutrition Laboratory, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad. Before the start of the experiment, fingerlings were kept in glass aquaria to acclimatize to laboratory conditions and basal feed was given for one week. The experimental duration was 4 months.
Experimental Design
The experiment was carried out in 5 glass aquaria with 3 replicates for a period of 4 months and fingerlings of the same size of L. rohita were kept as 10 fish per aquarium. Fingerlings of L. rohita were cultured under intensive condition and 5 experimental groups were made. Control group (T0) was fed with basal diet. The remaining aquaria known as experimental groups constituted of 30% and 50% plant protein mixture. Four diets were formulated containing T1 with 30% plant protein mixture with 2% probiotics, T2 with 30% plant protein mixture with 4% probiotics, T3 with 50% plant protein mixture and 2% probiotics and T4 with 50% plant protein mixture and 4% probiotics. Fingerlings were given 30% and 50% plant protein mixture along with the addition of 2% and 4% probiotics that is Bacillus species. Physico-chemical parameters and growth parameters such as weight gain, length gain, feed conversion ratio and specific growth rate were measured on weekly basis. Regular water exchange was ensured to maintain water quality.
Experimental Feed
All feed ingredients that include fishmeal, soybean meal, sunflower meal, cotton seed meal, wheat flour, wheat bran, rice bran, soy oil, vitamin mineral premixes, lysine, methionine and probiotic were bought from local market. The ingredients were weighted and finely grounded. Five experimental feeds were prepared and designated as T0, T1, T2, T3 and T4. T0 was control group and contained 100% fishmeal while T1 contained 30% plant protein mixture and 2% probiotics, T2 contained 30% fishmeal replacement with plant protein mixture along with 2% probiotics, T3 contained 50% plant protein mixture with 2% probiotics and T4 contained 50% plant protein mixture with 4% probiotics. Feed was given two times a day @ of 6% of fish body weight.
Feed Processing
Fishmeal, soybean meal, cottonseed meal, sunflower meal, wheat flour, wheat bran, rice bran, methionine, lysine, vitamins and minerals were purchased from local market. They were taken in required amount and following step were followed for preparing the feed.
Soybean, Sunflower and Cotton Seed Processing: Soybean, sunflower and cotton seed were bought from the local market of Faisalabad. These seeds were prepared by cleaning them and soaking them in water to get rid of some anti-nutritional elements (ANFS). The seeds were then put in an oven set at 12 0C. All the ingredients were weighed and grind in similar particle size by using mortar and pestle and were mixed by using mixer to improve the palatability. Vitamins and minerals were also mixed in small quantity. Soy oil was added into the feed and mixed for 5 minutes. Powdered of Bacillus subtilis and Bacillus pumilus probiotics were bought from a local store in Faisalabad. To promote growth and lower the risk of illnesses and infections, they were completely mixed in with the fish feed after being added in powder form (Figure 1). The above-mentioned table that is (Table 1) is covering the whole feed ingredients that can be used in an experimental system including the experimental feed and research referred feed ingredients as well. They include rice bran, wheat bran, wheat flour, plant protein mixture, fishmeal and probiotics as well. After weighing all the ingredients, the processing of feed was done. The processing of feed was performed to improve the digestibility of ingredients. Feed was kept in air tight jar and named as T0, T1, T2, T3 and T4 (Figure 2).
Note: T0: Control group (100% fishmeal) T1: 30% plant protein mixture and 2% probiotics T2: 30% plant protein mixture and 4% probiotics T3: 50% Plant protein mixture and 2% probiotics T4: 50% Plant protein mixture and 4% probiotics
Estimation of Growth Parameters: Growth parameters such as specific growth rate, body weight gain (g), length gain (cm), feed conversion ratio (FCR) and Specific growth rate (SGR) was monitored on weekly basis. The following procedure were used to determine the growth performance.
Weight Gain: Body weight gain was calculated in grams by using following formula:
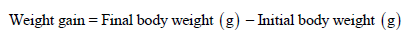
Length Gain (cm): During the whole trial, fish were collected for the measuring fish length. Length gain was calculated in (cm) unit as following:
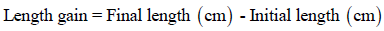
Specific Growth Rate (SGR): It was determined per week. After every week, the final weight was subtracted from the initial weight and the data obtained this way was divided by number of the days (seven days) and then multiplied by 100 (Figures 3 & 4).
The formula for the calculation of SGR is given below:
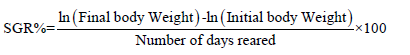
Where: ln= the natural log
Feed Conversion Ratio (FCR): Feed conversion ratio was determined per week. The formula for the calculation of FCR is given below:
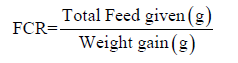
Physicho-Chemical Parameters: To maintain water quality parameters for water quality must be determined. Weekly measurement of pH, temperature, dissolved oxygen and total hardness were taken throughout the experiment. Temperature and dissolved oxygen measurement were made using the HANNAH HI-9147. Aquarium water’s pH was measured using a digital meter. The daily mentioned procedure was used to calculate total hardness.
pH: An important factor is the pH of natural water. The pH scale ranges from 0 to 14, where a value of 7 or less points to acidic water and a value of 7 or more points to alkaline water. The majority of fish species prefer a pH between 6 and 9. Fish often benefit from pH levels that are on the somewhat alkaline side. The pH meter (HI-8424) was used to test pH (hydrogen ion concentration scale) (Figure 5).
Temperature: One of the key elements influencing the growth of fish is phytoplankton, zooplankton and temperature. The temperature of the water can affect a lot of aquatic organisms. If it rises, fish development will slow down because rising temperatures cause water’s oxygen content to drop (Figure 6).
Dissolved Oxygen: All aquatic life depends on dissolved oxygen. Including the organisms that digest contaminants created by humans. Since oxygen is soluble in water, it will equilibrate with the ambient oxygen when present water. The more temperature rises, the less soluble oxygen becomes. After setting its range in ppm unit, the dissolved oxygen meter (HI-9147) detected dissolved oxygen. The sensor of the meter was dipped into the water tank to measure the dissolved oxygen (Figure 7).
Total Hardness: The total hardness of Ca2+ and Mg2+ ions in natural water, represented as CaCo3 is referred to as total hardness. A 50ml sample of water was placed in an Erlenmeyer’s flask and pH was kept between 12 and 13 by adding the proper amount of buffer solution. Eriochrome Black T (EBT) indicator was added to the reaction mixture mixed and titrated against Ethylene Diamine Tetra Acetic Acid (EDTA) (0.01N) to reach the end point, which was blue in color. The following formula was used to determine the overall hardness:
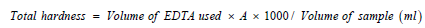
Total Alkalinity: The overall concentration of carbonates or bicarbonates in water bodies is known as total alkalinity. The water body also contains silicates, hydroxides, phosphates and ammonia among other bases. Bicarbonates are present when the pH of water is below 4.5 and the color of the water changes to yellow. To calculate the total alkalinity of water the following approach was employed. To prevent denaturation, a water sample was collected and immediately analyzed in a polythene bag. In Erlenmeyer’s flask, 20ml of water were obtained and 0.1ml of methyl orange were added to the water. The reaction mixture was stirred and it was then titrated against 0.1N of sulfuric acid to reach the end point which was faintly orange color. Total alkalinity was measured by the following formula:
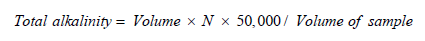
Intestinal Morphology: After the end of the feeding trial, intestine mid-section of five fish from each replicate was excised carefully. These sections were then preserved in 10% formaline solution for 24 hours. Then, dehydrated in ascending grades of alcohol and cleared in xylene. Samples was embedded in parafine wax by using microtome, 5µm sections were cut. After that the staining of sections was done. Now, examined the sections microscopically and photographs were taken. For statistical analysis, Qupath software was used to measure the mean villus height from base to top (Ibrahim, et al., 2021). Absorption surface area was calculated according to Mohammady et al. (2021).
Statistical Analysis: The statistical design of this experiment was ANOVA under Completely Randomized Design (CRD) and Tukey’s test was used to analyze the data statistically. Growth and Intestinal morphology were analyzed by comparing the mean values.
This research was carried out to determine the effect of partial replacement of 30% and 50% plant protein mixture on growth production and intestinal morphology of Labeo rohita. This experiment was conducted at the Fish Nutrition Laboratory, Department of zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad. Trial duration was four months. During this study period, assessment of replacing fishmeal with 30% and 50% plant protein mixture supplemented with 2% and 4% probiotics i.e. Bacillus sp. was carried out.
The results of this research are evaluated as following:
• Evaluation of growth performance
• Evaluation of intestinal morphology
• Physico-chemical parameters
Growth Parameters
Growth refers to the increase in size and weight of fish over time. It is an important matric in fisheries and aquaculture and it is the measurable physical characteristics of species. This study was held to evaluate the growth and intestinal morphology of L. rohita by replacing 30% and 50% fishmeal by plant protein mixture for 16 weeks. On weekly basis the growth of L. rohita was calculated in terms of length gain (cm), weight gain (g), Feed conversion ratio and Specific growth rate.
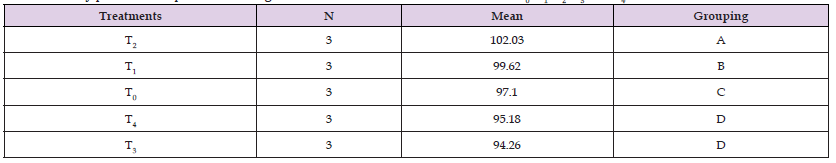
Measurement of Average Weight: Weight was measured in the first week of trial as initial weight. The initial weight of rohita for T0, T1, T2, T3 and T4 were recorded as 13.2g, 13.3g, 12.98g, 12.84g and 12.93g respectively. After the replacement of 30% and 50% fishmeal with plant protein mixture, at the end of the trial, fish gain weight as the final body weight for T0, T1, T2, T3 and T4 were recorded as 97.1g, 99.62g, 102g, 94.26g and 95.18g respectively. Similarly, weight gain was also recorded in both experimental and control group and statistically significant (Tables 2-5) (Figure 8).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Note: *= Significant (P<0.05) * NS=Non-significant (P>0.05)
Table 5: Tukey pairwise comparison for Weight Gain of L. rohita under the influence of T0, T1, T2, T3, and T4
Measurement of Average Length: Before stocking in the aquarium length measurements were done. T0, T1, T2, T3, and T4 have average length of 7.3cm, 7.5cm, 7.6cm, 6.89cm and 6.99cm respectively. At the end of the experiment this length increased to 20.21cm, 21.4cm, 21.9cm, 19.2cm and 19.7cm for T0, T1, T2, T3 and T4 Similarly, length gain was also recorded in both experimental and control group and statistically significant results were observed (Tables 6-9) (Figure 9).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Note: *= Significant (P<0.05) * NS=Non-significant (P>0.05)
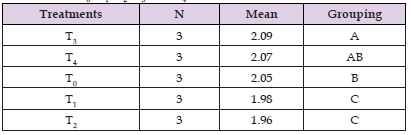
Feed Conversion Ratio: The feed conversion ratio (FCR) is the quality of feed needed to produce 1kg of fish. Feed conversion ratio was calculated on weekly basis throughout the whole research period. Feed conversion ratio is a ratio that measures the efficiency with which animal body convert the feed into the desired output. There were fluctuations in feed conversion ratio within weeks throughout the experiment. Mean value of feed conversion ratio in T2 is 1.96 and in T1 is 1.98 which is less than control group T0 and other experimental groups like T3 and T4 (Tables 10-13) (Figure 10).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Note: *= Significant p<0.05) * NS=non-significant (p>0.05)
Table 13: Tukey Pairwise Comparison for FCR of L. rohita under the Influence of T0, T1, T2, T3, and T4 .
Specific Growth Rate: Growth is a crucial indicator in aquaculture and fisheries. Specific growth rate express growth as the intuitively understandable percent change in size per unit of time. Specific growth rate showed the efficiency of feed to improve the growth performance. SGR calculated on weekly basis. Every week weight was calculated and then subtracted from the initial weight, then divided by number of days and then multiplied with 100. Higher SGR value in T2 was 2.07 while lowest was found to be 1.98 in T3 and specific growth rate in T0, T1 and T4 was 2.02, 2.03 and 2 respectively (Tables 14-17) (Figure 11).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Note: *= Significant p<0.005) * NS=non-significant (p>0.005)
Intestinal Morphology
Intestinal morphology refers to the study of the structure and organization of the intestines, including small and large intestine. Intestinal morphology is studied to understand how the gut function, how the nutrients are absorbed, and how the gut responds to different diets and diseases. Plant protein in the diet of fish can positively affect intestinal morphology by increasing villi height and width. Plant protein mixture can also stimulate the growth and development of intestinal cells and it also promote healthier gut microbiota. Gut microbiota plays a crucial role in the development of the gut by regulating the immune system and reducing inflammation and shaping the gut morphology and function, and also producing metabolites that promote gut health.
Villus Height: Villi height refers to the height of the small, finger-like projections (villi) that line the walls of the small intestine. These villi play crucial role in nutrient absorption. These villi increase the surface area of the small intestine, allowing for more efficient absorption of nutrient from food. The height of villi can vary depending on factors such as the individual’s health status and dietary habits. Increased villi length indicates a greater surface area for nutrient absorption. The unit for the measurement of villus height is µm. The villus height calculated for treatments T0, T1, T2, T3 and T4 were as 160.1, 173.3, 177.2, 169.2 and 170.2 (µm) respectively (Tables 18-21) (Figure 12).
Note: T0: Control group (100% fishmeal) T1: 30% plant protein mixture and 2% probiotics T2: 30% plant protein mixture and 4% probiotics T3: 50% Plant protein mixture and 2% probiotics T4: 50% Plant protein mixture and 4% probiotics
Note: *=Significant (p<0.05) * NS=non-significant (p>0.05)
Villus Width: Villi width refers to the thickness of the villi. Villus width is similar to its height. It is typically measured in µ It refers to the diameter or thickness of finger like projections that make up the villi in the linning of the small intestine. This measurement helps to characterize the overall size and structure of the villi, which play a crucial role in increasing the surface area of the small intestine for efficient nutrient absorption. Wider villi indicate a greater surface area for nutrient absorption and a healthier gut. The villi width calculated for treatments T0, T1, T2, T3 and T4 were as 81.1, 92.5, 97.2, 86.7 and 88.9 (µm) respectively (Tables 22-25) (Figures 13 & 14).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Note: *= Significant p<0.005) * NS=non-significant (p>0.005)
Physicho-Chemical Parameters
Physicho-chemical parameters include the chemical and physical properties of water describing the water quality. Physico-chemical parameters such as temperature, pH, DO, total alkalinity and total hardness were monitored on weekly basis throughout the 16 weeks’ trial period.
Temperature: The growth and survival of all living things are directly impacted by temperature, which is an important factor. Fish experience stress due to extreme temperature changes, which slow down their rate of growth. Temperature is basically important for its effect on the chemistry and biological activities of organism in water. Temperature is known to influence in the determination of other factors like dissolved gases, pH, conductivity and various forms of alkalinity. The rate of chemical reactions generally increases at higher temperature. Warm water holds less dissolved oxygen than cool water therefore, it is not sufficient for the survival of different species of aquatic life. Throughout the trial temperature was regularly monitored by using HANNA-HI 9143 temperature meter. Temperature with a range of 26.9-320C was recorded (Table 26) (Figure 15).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Dissolved Oxygen (DO): Dissolved oxygen is a term used to described the quantity of free, non-compound oxygen in water. Due to its impact on the aquatic life, it is a crucial factor in determining the quality of water. Changes in dissolved oxygen concentration directly affect fish growth and survival. It provides information about the water’s quality and is crucial to the efficiency of organism’s metabolic processes. Dissolved oxygen was observed on weekly basis. Throughout the course of the experiment, a range of 5.8-5.1mg/L was noted. The level of DO decrease with the increase in temperature. The low dissolved oxygen concentration observed during summer could be ascribed to the higher salinity of the water and higher temperature (Table 27) (Figure 16).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
pH: The amount of H+ ions and the degree of acidity or alkalinity in a solution is measured by pH. The most crucial factor in determining how acidic or basic water is pH. The solubility and toxicity of chemicals and heavy metals in water can also be impacted by pH. Since it has a pH of 7, pure water is neither acidic nor basic in nature. In aquatic species, pH fluctuations can cause toxicity, sluggish development and no reproduction. While certain aquatic organisms can survive in water with pH values outside of this range, most prefer a pH range of 6.5 to 9.0. Every week during the trial, the pH was measured. During the trial time, the control and experimental groups showed a range of 7.5-8.2, which was within the ideal range (Table 28) (Figure 17).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Total Hardness: The total hardness of a solution is determined by the presence of Ca2+ and Mg2+ Water’s pH and total hardness are directly related. The minerals in hard water function as a buffer in the water body to prevent the water from becoming too acidic. As a result, the outcomes will be more alkaline, raising the pH of the water. Cloudy water will come from a significant increase in the calcium level in the water body. This may result in the water pump becoming clogged and blocked. The ideal level is crucial for maintaining the fish’s health and behavior as well as providing a good, suitable environment. A range of 155-173mg/L was observed at the end of the trial (Table 29) (Figure 18).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
Total Alkalinity: Alkalinity is measurement of the water body's capacity to neutralize acids and bases and to preserve a pH level that is generally stable so it acts as a stabilizer for pH. The ionic concentration of carbonates, bicarbonates and hydroxide in a body of water is known as total alkalinity. Other bases including silicate, ammonia and phosphate are also present in addition to these. It is often expressed in units of milligram per liter (mg/L) or parts per million (ppm) of calcium carbonate (CaCO3). A range of 175-215mg/L was observed (Table 30) (Figure 19).
Note: T0: 100% fishmeal T1: 30% plant protein mixture with 2% probiotics T2: 30% plant protein mixture with 4% probiotics T3: 50% plant protein mixture with 2% probiotics T4: 50% plant protein mixture with 4% probiotic
The aim of this study was to determine the effect of 30% and 50% replacement of fishmeal with plant protein mixture on growth and intestinal morphology of Labeo rohita. During this study, the growth parameters like weight gain, length gain, FCR, SGR and survival rate were measured. Intestinal morphology like villus length and villus width were also measured at the end of the 16 weeks’ trial. Control diet was given to the control group T0. Experimental diet contain 30% plant protein mixture with 2% and 4% probiotics was given to the T1, T2, and 50% plant protein mixture supplemented with 2% and 4% probiotics was given to T3 and T4. Fish meal is the main protein source in aquaculture feeds due to its high digestibility and palatability and it has balanced amino acid composition (Riche, et al. [16]). With the explosive growth of aquaculture sector fishmeal is becoming more and more expensive. For successful aquaculture a low cost of production of feed is essential that is nutritionally balanced. Global research is prioritizing to find the substitute protein sources for fishmeal in diets (Ping, et al. [31]). Studies on plant protein sources, such as cottonseed meal, sunflower, and soybean meal have been conducted in recent decades. Plant based protein sources have been generally used to substitute dietary fish meal in fish feed because they are continually available and environmentally safe (Elumalai et al., 2023). Fish meal has always been a key ingredient in feed formulations. As a result of its balanced amino acid composition, palatability and growth potential, fish meal is the preferable ingredient in fish feed.
However, a rise in the price of fish meal has compelled fisheries experts to use various substitute sources in its stead. The substitution of fish meal with the mixture of plant protein sources in aquatic feed has become a prominent trend in aquaculture industry (Jannatullah, et al. [32]). Due to the rise in the price of fish meal from fish feed, lead the experts for the substitution of fishmeal with plant protein sources (Radhakrishnan, et al. 2016). It has already been tried to replace fish meal sources with minimum supplemental amino acid (AA) without influencing feed consumption and growth performance of fish (Cabral, et al. 2011). During this trial, physico-chemical parameters such Temperature, Dissolved oxygen, pH, Total alkalinity and Total hardness were recorded on weekly basis. In this study the value of average dissolved oxygen was 5.46 mg/L for T0 group, 5.43 mg/L for T1, 5.43 mg/L for T2, 5.45 mg/L for T3 and 5.4mg/L for T4 group. And means of total hardness for T0, T1, T2, T3 and T4 were as 163.98, 163.93, 164.52, 164.7 and 165.74 mg/L respectively. Average values for total alkalinity for T0, T1, T2, T3 and T4 were as 192.41, 194.19, 192.35, 192.88 and 192.66 mg/L respectively. The mean values of pH for T0, T1, T2, T3 and T4 were as 7.78, 7.79, 7.81, 7.83 and 7.76. The average values of temperature for T0, T1, T2, T3 and T4 were as 30.03, 30.07, 30.05, 30.2 and 30.19 oC. In this trial, the growth production of fish was evaluated on weekly basis in which all growth parameters such as weight gain, length gain, feed conversion ratio and specific growth rate were observed.
The maximum value of weight gains and length gain was recorded in experimental group; T2 (30% plant protein mixture with 4% probiotics) which was 102.09g±0.3 and 21.9cm±0.12 respectively while minimum weight gain and length gain values was recorded in T3 (50% plant protein mixture with 2% probiotics) which was 94.26±0.3 and 19.2±0.15. Statistically significant results were recorded in weight and length gain and these results were similar with findings of (Zhou, et al. [33]). The similar results were also given by Lee et al. (2016) which indicated that 20% fishmeal can be replaced with soybean meal without having negative effect on growth performance of juvenile rockfish. At the end of this study, after observing final results it was suggested that fishmeal can be replaced with 30% and 50% plant protein mixture as supplemented with 2% and 4% probiotics without any non-significant effect on growth performance and intestinal morphology of Labeo rohita. Specific growth rate (SGR) used to measure percent increase in fish weight over time. In the present trial, the higher SGR value (2.07±0.06) was observed in experimental group T2 in which replacement level was 30% fishmeal to plant protein mixture with 4% probiotics as compare to control group. The SGR value in T0, T1, T3 and T4 were 2.02±0.05, 2.03±0.06, 1.98±0.06 and 2±0.06 respectively. Feed conversion ratio (FCR) is related to amount of feed given to the fish and weight gain by consuming that feed. Feed conversion ratio decreases with the increase in weight of fish.
FCR values in T0, T1, T2, T3 and T4 were 2.05±0.12, 1.98±0.12, 1.96±0.13, 2.09±0.13 and 2.07±0.12 respectively. Statistically significant results were observed by feed conversion ratio (FCR) and specific growth rate (SGR) and were in similar trend with the outcomes of (Zhou, et al. [33]). Control group showed less growth production with 100% fishmeal as compare to T2 and T1 experimental groups which contain 30% plant protein mixture as supplemented with 2% and 4% probiotics. Experimental groups T3 and T4 show less growth as they contain 50% plant protein mixture as supplemented with 2% and 4% probiotics. To maintain optimal growth and health, it is critical to understand and meet each fish specie’s dietry protein and particular amino acid requirements. As plant protein mixture is increased in the diet of fish, it exhibited a negative effect on the weight and length of Labeo rohita. A similar study was also performed by (Xu, et al. [34]), in which he analyzed the effect of different inclusion of plant protein mixture on growth of fish. Five experimental diets were created to substitute fishmeal at different percentage of mixed plant protein: 0% plant protein mixture for control group, 25% for T1, 50% for T2, 75% for T3 and 100% replacement for T4 group. The weight increase, specific growth rate and protein efficiency ratio were significantly high up to 25%and 50% replacement, however these parameters were decreased by replacement above 50% plant protein mixture. The intestinal morphology is essential for overall well-being of fish (Abdel Rahman, et al. [35-40]).
It is studied to understand the structure and function of the intestine, which is important for nutrient absorption and immune function. A diet containing plant protein with supplementation can significantly impact intestinal morphology. Absorption of nutrients occurs through the intestinal villi and the epithelial cells. Intestinal villi play a key role in the digestion and absorption of nutrients (Hu et al. 2016). Both the length and thickness of the intestinal villi are essential indicators for assessing the absorbability of the small intestine. This research trial was conducted to investigate intestinal morphology of Labeo rohita in which villus length and width were observed. Intestinal morphology (villus height and villus width) was observed at the end of experimental trial in all the treatments in comparison with the corresponding initial values. The maximum value of villus length was recorded in experimental group T2 (30% plant protein with 4% probiotics) which was 177.2±0.26 while minimum villus length was recorded in T3 (50% plant protein mixture with 2% probiotics) which was 169.2±0.3. These results were statistically significant. The maximum value of villus width was recorded in experimental group T2 (30% plant protein with 4% probiotics) which was 97.2±0.2 while minimum villus width was recorded in T3 (50% plant protein mixture with 2% probiotics) which was 86.7±0.6. The results were statistically significant. These results were also similar with outcomes of (soltan, et al. [17, [41-55]) which indicate that plant protein mixture at this level has no negative effect on the intestine of L. rohita. Fish intestine plays an important role in the digestive and absorptive functions of the alimentary tract, thereby showing a significant effect on fish nutrition and growth (Bhujel et al. [56-78]).
The study evaluated the impact of varying levels of plant protein mixture (30% and 50%) and probiotics on the growth performance and intestinal morphology of Labeo rohita. These findings indicate that incorporating plant protein mixtures and probiotics in the diet of Labeo rohita can be a promising strategy to improve aquaculture sustainability by reducing reliance on fishmeal while maintaining or enhancing fish growth and gut health. Further studies are warranted to optimize the inclusion levels of plant proteins and probiotics, as well as to investigate their long-term effects on fish health and productivity.


