Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Dursa Miressa1, Gemechu Gure2 and Adugna Girma3*
Received: August 17, 2024; Published: August 27, 2024
*Corresponding author: Adugna Girma Lema (DVM,MSc), Yemalogi welel Woreda livestock and fisheries development office. Kellem Wollega, Oromia, Ethioipa
DOI: 10.26717/BJSTR.2024.58.009149
A cross sectional study was conducted with the objectives of determining the prevalence and risk factors associated with sheep major gastrointestinal nematodes in and around Abayi Chomen district, Horo Guduru Wallaga part of Ethiopia from November, 2021 to May, 2022. Out of 384 sheep randomly sampled in the study area, 384 sheep were examined for GIT nematode infections using fecal flotation technique. Anoverall prevalence of gastrointestinal nematode of examined animal in the study area was 297(77.3) %. Among the samples from sheep were detected positive for gastrointestinal nematode parasites. Out of types of egg identified, Strongyle 116(30.2%) was the dominant followed by Mixed type of eggs 128(10.2 %), Strongyloide 28(7.3%) and Trichuris 25(33.3%). Among the risk factor considered in this study area the study revealed that no statistically significant difference (p>0.05) was found in prevalence between Sex and species of examined animal. Age and body condition of the animals were showed statistically significant difference (p<0.05). The Sex wise prevalence was 171(72.4%) and 126(78.5%) in male and female animals respectively while that of age was 121(85.5%), 176(72.4%) in young and adult animals respectively. Body condition score infection rate was 72%, 66.1% and 95.4% in poor, medium and good body conditions respectively. Based on the McMaster EPG result, the study animals were classified as light (13.3%), moderate (37.2%) and Severe (26.8%) infection. The majority of examined animal had the EPG count in average of less Than 900.The study shows that the GIT nematode parasites were major important health problem and impact on the production of sheep in the study area. Therefore, special consideration should be taken on the management of sheep in poor condition to reduce burden of gastrointestinal nematodes, strategic deworming of sheep using broad spectrum anti helminthics should be practiced and further studies on economic losses and epidemiology of GIT parasites of sheep should be conducted. Therefore, effective strategic treatment and public awareness creation should be instituted in the study area.
Keywords: Abayi Chomen; Antihelminthics; Gastrointestinal Nematodes; Sheep; Prevalence
Livestock systems in developing countries are diseases, characterized by rapid change, driven by factors such as population growth, increases in the demand for stock products as incomes rise and urbanization. Livestock currently contribute about 30 percent of agricultural gross domestic product in developing countries, with a projected increase to about 40 percent the by 2030 and is becoming the fastest-growing countries. Sub-sector of agriculture (Basaznew, et al. [1]). Gastrointestinal parasites are the major animal health constraints in sheep and goat production and contributing loss in productivity and economy. In the present study an overall prevalence of GIT parasites were high with occurrence of mixed infection. The predominant GIT parasites identified were Strongyle spps, Trichuris spps, strongyloids spps. Risk factor like ages, sex, body condition and production system were found determinant factors for the occurrence of GIT parasites. From the above findings and conclusions, strategic use of anthelmintics and good management should be practice to control the gastrointestinal parasites infection with further study on Seasonal variation and burden of parasites in the study area (Olifan, et al. [2]). Gastrointestinal parasite infections have greater impact in Ethiopia due to the availability of a wide range of agro-ecological factors suitable for diversified hosts and parasite species. Small ruminants are among the major economically important livestock in Ethiopia; in which there are 23.62 million sheep and 23.33 million Goats, playing an important role in the livelihood of resource poor farmers and provide a vast range of products and services such as meat, milk, skin, hair, horns, bones, manure and urine, security, gifts, religious rituals and medicine. Sheep and goats are particularly important resources for their owners, because they require smaller investments, have shorter production cycles, faster growth rates and greater environmental adaptability than cattle. Therefore, they form an important economic and ecological niche in all agricultural systems throughout the country. However, diseases often prevent them from attaining optimum productivity (Surafel, et al. [3]). However; diagnosis of gastrointestinal nematode infections plays a major role in investigating parasite epidemiology.
The ante mortem diagnosis of nematode infections in livestock has been based on the detection of nematode eggs or larvae in the faeces by microscopic examination using the methods of flotation and/or larval culture. Although a direct fecal smear can be examined, the mere presence of parasite eggs is not helpful in determining the parasite load of an animal or animals. Quantifying of the egg per gram of feces is the best way of estimating parasite loads (Basaznew, et al. [1]). The direct victims caused by these helminth parasites are attributed to keen illness and death, early butcher and elimination of some parts at meat scrutiny. Indirect losses owners, because they require smaller investments, have include the reduction of productive potential such as decreased growth rate, weight loss in young growing animals and late development of slaughter stock. The prevalence of GIT parasites, the genera of helminth parasites involved, species and the severity of infection also vary considerably depending on local environmental. Conditions such as humidity, temperature, rainfall, vegetation and management practices (Surafel, et al. [3]).Therefore the objective of this study was to determine the prevalence of major GIT nematode helminth parasites and associated risk factors in sheep. Gastrointestinal worms in particular, are often a primary animal health issue on many farms and ranches. Losses caused by heavy parasite burdens are both direct, in terms of death, poor gains and reproductive inefficiency and indirect, stemming from increased susceptibility to secondary infection and greater labor needs. Major element in ensuring the sustainability of sheep and goat production is currently achieved by the use of anthelmintics (Basaznew, et al. [1]). The high occurrence of GI parasites in sheep and goats might be related to hygienic practices on farms and management of grazing land (Shwe, et al. [4]). When the large Livestock population of Ethiopia is compared with its economic benefits, it remains less because of prevailing diseases, poor nutrition, poor animal production systems, reproductive inefficiency, management constraints and awareness of owners. Major problem of animals in the world. These nematode infections affect the health of millions of animals, causing a huge economic loss in livestock farming (Ahmed M, et al. [5]).
The objectives of this study were:
a) To determine the prevalence and its associated risk factors of Gastrointestinal Nematodes parasites of Sheep in and around Abayi Chomen district Horro Guduru Wallaga, Ethiopia
b) To determine level of infestation of gastrointestinal nematodes of sheep in selected study area.
Study Area
Abayi chomen is located in the western oromia of Ethiopia in Horro Guduru Wallaga Zone, Oromia Region State about 290 Kilometers from capital city Addis Abeba. A study was conducted from November 2021 to may, 2022 to determine the prevalence of major gastrointestinal nematode parasites in sheep in Abay chomen district of 6(six) kebeles was selected to collect the samples (Fincha 02, Kolobo, Ganji Haro, Jare, Achane and Ganji Ketala). The District lies 80% high land and Fincha Lake, Amerti and Nashe (FAN) project dam was found in this district. The majority of the population depends on subsistence farming. The altitude ranges from 1700 to 2700 matter above sea level. The area receives a bimodal annual rain fall which a range between 700-1160 mm. Communal grazing is in practice in the area.
The population of the Abayi Chomen 73,907 in number and veterinary infrastructure of the Abayi chomen area 1 B type veterinary clinic,1 C type veterinary clinic, 5 D type clinic and number vets are 3 DVM. 3 BVSc, 8 animal health assistant workers (Abayi Chomen agricultural office, 2022).
Description of Study Population
The livestock population of the district is comprised of 71,053 cattle (70631 local and 422 cross breed), 6238 sheep, 8728 goats, 11 mules, 6461 donkeys, 357 horses, 31390 poultry (25732 local and 5658 exotic) (source; Abayi Chomen Agricultural office, 2020)
Study Animals
The study animals was local breed of sheep (Horro Breed), kept under traditional extensive management system. From the individual used to select study animals. Age, sex, species and body condition was considered as risk factors for the occurrence of major gastrointestinal nematodes in sheep.
Sample Collection and Examination Procedure:
Collected fecal samples were put in the sampling bottle containing 10% formalin and necessary information was labeled. The collected samples were transported to Fincha Veterinary type “B” clinic laboratory and were stored at refrigerated temperature (4°C) until processing. In the laboratory, fecal samples was examined for the detection of nematode eggs using standard procedures of simple flotation techniques (Annex 2) for screening of study animals for nematodes presence and McMaster techniques (Annex 3) to identify and counting of GIT nematodes following the standard procedures (Hendric [6]).
Study Design and Sample Size Determination
A cross-sectional study design was used to determine the prevalence of gastrointestinal nematodes of sheep in Abayi Chomen district based on parasitological examination of fecal samples. Simple random sampling technique was followed to collect faces from the individual used to select study animals. Age, sex, species and body condition was considered. The total sample size was calculated based on the predetermination of the following parameters: a 95% level of confidence, 5% desired level of precision and 50% expected prevalence according to Thrusfield [7] since, there was no similar study done previously on the study area. In all the analyses, confidence level will be held at 95% and P<0.05 set for significance.
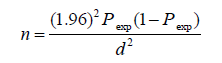
Where: n = required sample size; Pexp = expected prevalence; and d = desired absolute precision. The sample size was calculated based on 50% expected prevalence and with 5% absolute precision. So the calculated sample size was:
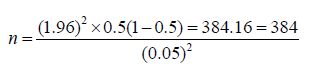
Data Management and Analysis
The collected data from field and laboratory investigation was coded in to appropriate variables and entered in to Microsoft excel work sheet. All statistical analysis was performed using statistical software packages for social science (SPSS). The prevalence was calculated by dividing the number of positive animals by the total number of animals examined (384) and times 100. Percentage (%) and Chi-square (x2) were calculated to measure prevalence and association between prevalence of the parasite and species of animals, age, and sex and body condition score respectively.
Prevalence of gastrointestinal Nematode parasites of sheep examined
Our 384 sheep examined coprologically for gastrointestinal parasite eggs 297(77.3%) where found positive. There was no statistically significant difference in prevalence of gastrointestinal parasites between sexes (p<0.05). Body condition was found to be a risk factor for GIT parasites prevalence where the poor body condition sheep had higher prevalence of the parasites 126(95.4) whereas the lowest prevalence was recorded in medium (66.1) body condition score with a statistically significant difference (P>0.05) (Table 1).
Prevalence of gastrointestinal nematode parasites per the study sites
All the sheep examined coprologically in six kebeles, samples from Jare showed slightly higher parasitic infestation (85 %) as compared to other sites. The difference between study kebeles was found to be statistically significant (p>0.05) (Table 2).
Prevalence of Gastrointestinal Nematode Parasites Using Different Laboratory Techniques
The total 384 sheep examined, 297(77.3%) were found infected with different types of gastrointestinal parasites 116(30.2%) where infected with strongyle species 25(33.3%) were infected with trichuris 28(7.3%) were infected with strongyloides species and 128(10.2%) were infected with mixed types according to their descending order of prevalence (Table 3). Individual animal may harbor more than one parasite.
Quantitative Faecal Examination Finding
In this study, evaluation was done to determine the effect of sex on disease prevalence.Females and males were found to be infested with a slight variable degree but without statistically significant variation (P<0.05). Accordingly, slightly higher prevalence of GIT nematode infection was observed in female animals (78.75%) as compared to males (76.3%) (Table 4).Young and adult animals were found to be infested with a prevalence of 85.8% and 72.4%, respectively with no statistically Significant difference (P<0.05) between age of examined animal (Table 5 ). Poor body condition score sheep have higher prevalence record (95.4%) followed by medium body condition score (66.1%) whereas the lowest prevalence was recorded in good (72%) body condition score with a statistically significant difference (P>0.05) (Table 6). A total of 297 fecal samples that were positive by qualitative parasitological techniques were positive to nematode species by qualitative floatation technique were subjected to EPG count using MC-Master egg counting technique. Accordingly, 51(13.3%), 143(37.2%), 103(26.8) sheep were found to be lightly, moderately and severe infested, respectively. slightly significant difference was observed in the EPG count across sex groups (p>0.05). Most of the infected sheep had a faecal egg count in a range 50-799 light, 800-1200 moderate and >1200 severe infestation.
The gastrointestinal nematodes of sheep are one of the major important parasitic diseases that reduced the productivity of sheep raised by farmers using traditional management system in and around Abayi Chomen District. The coprological examination done for this study using different techniques revealed an overall gastro-intestinal helminthes infestation with prevalence of 77.3% (n=384) The coprological examination performed for this study revealed the existence of nematodiasis in small ruminants with an overall prevalence. This finding is lower than the results of other surveys in small ruminants in Western Regassa, et al. [8-12]), Southern Ethiopia. The decrease in the GIT nematodiasis prevalence in the present study compared with the other studies in the country could be due to the existence of unfavorable climatic or environmental factors that could support prolonged survival and development of infective larval stage of most nematodes Amenu[13] and Rossainga [14] Different coprological examination done revealed that sheep were affected by strongyle species, Trichuris species, Strongyloides species.this finding is in harmonious with reports of previous studies conducted in Ethiopia by Fikru, et al. [15], Hailelul [12] and Tefera, et al. [16]. Among the different parasites identified from the faeces of sheep, the prevalence of strongyle species counted for (39.06 %), Trichuris species (8.42%), Strongyloides species (9.43%) and mixed types are (43.10%). In this study, the strongyle species were identified in general terms, since there eggs were not differentiated easily to genus level Van Wyk, et al. (2004).
The current prevalence of gastrointestinal Strongyles agrees with reports of previous studies conducted in different parts of Ethiopia by Tigist [17,18] and Ragassa et al. [8] who reported prevalence of 56.6%, 66.6% and 70.2%, respectively. The prevalence report (43.3) by Tesfaye [11] was slightly lower than the current finding. This difference in prevalence rate in different parts of the country might be attributed to the difference in agro ecology and variation in management practices of animals. The prevalence of Strongloides species in the present study was 6.1% which agrees with the report from Bedelle by Tefera, et al. [16] and from Debre Zeit by Tigist [17] who reported the prevalence of strongyloides species as 13.04% and 8.2%, respectively. This finding was lower as compared to 45.22% from Eastern part of Ethiopia by Abebe and Esayas [18]. This variation might be correlated with difference in agro ecology.The prevalence of Truchuris species was 7.3% and this finding was in the line with work of Bersissa, et al. [19-20] and Ragassa, et al. [8] with prevalence of 7.9%, 5%, 3.3%, and 4.5, respectively. The present finding however was compared to 30.25% from Eastern parts of Ethiopia by Abebe and Esayas [21]. This variation in prevalence might be attributed to difference in agro ecology and management practices of the study area.The present study shows no statistically significant differences in the prevalence of GIT parasites between sex groups. This finding agrees with report by Ragassa [8], Assefa and sissay [22], Fikru, et al. [15], Getachew [23-24], Assefa and sissay animals did not show significant association with the prevalence of GIT parasites. This due to equal exposure of both sexes and they are from similar ecology. This finding disagrees with the work of Dagnachew, et al. [25-29] who reported higher prevalence of GIT parasites in females than males. These authors stated that female animals exposed to stress than male animals in different time such as during pregnancy and lactation which favors the egg output of parasites.Age wise observation revealed no statistically significant difference in infestation of GIT parasites. This finding agrees with reports from Gechi district of south west Ethiopia, Gambia and Semi-arid part of Kenya by Bikila, et al. [30-32] describing as GIT parasites affect both ages equally. The present finding disagrees with the finding with the finding of Fikru, et al. [15] Gamble and Zajak [33-35] that young animals are more susceptible to parasites infection than older one. The researcher justified the result that it could be because adult animals may acquire immunity to the parasites through frequent challenge and expel the ingested parasites before they establish infection. Young animals are susceptible due to immunological immaturity and immunological unresponsiveness. However, in this study the absence of significant difference in parasites infestation between ages of animals could be attributed to the small number of young animals used.
Difference in body condition score is statistically significant (p<0.05) with gastrointestinal parasite infestation such that shedding of parasites egg or oocyst were higher with poor body condition (96.15%) compared to moderate (66.36%) and good body condition (27.27%) animals. This finding agrees with report of Bisset, et al. [36,37] suggesting that well-fed animals develop good immunity that suppresses the fecundity of the parasites. This study also supports the report from Kenya by kanyari,et al. [34] who described animals with good body condition had lower prevalence of gastrointestinal parasites than those with poor body condition.There was statistically significant difference in prevalence of gastrointestinal parasite infection of sheep between study sites. Animals from Jare kebele showed slightly higher GIT helminthic infection (95.5%) as compared to other sites. Even if study sites have the same agro ecology this variation may be due to over stocking, starvation and frequent exposure to the fixed communal grazing lands that have been contaminated in the case of Jare kebele. This disagrees with report of Diriba and Birhanu [37] who reported no significant difference in animals with similar agro ecological conditions. It would be useful to have an agreed standard method for counting nematode eggs in faeces as there are several methods and several variations on the original McMaster technique. The details of one modified McMaster method accurate to 50 EPG was provided by (Coles, et al. [38-46]).
The present study conducted in and around Abayi Chomen district shows that gastrointestinal parasites were the most prevalent disease in the area affecting the wellbeing of the animals. Most predominant GIT parasites were mixed types, Strongyle, Trichuris, and Strongyloides species. All animals irrespective of age sex group were affected with parasites [47-53]. Body condition was considered as potential risk factors for the degree of infestation by mixed types and Strongyle parasites. The result of quantitative investigation reveals that majority of animals were moderately infested with Strongyle parasites. Majority of sheep in the study area were largely kept under traditional extensive management system which might increase chance of exposure to GIT parasites.Based on the above conclusion, the following recommendations are forwarded;
a) Awareness creation to the farmers in the study area on the effect of GIT parasites and its control
b) Strategic deworming of sheep using broad spectrum antihelminthics should be practiced.
c) Further studies on economic losses and epidemiology of GIT parasites of sheep should be conducted on the study area.


