Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Wakeel O Muritala1*, Oluseyi O.A Atanda2*, Muibat A Adeniran1, Taibat P Adesope3, Sherifat T Suleiman4, Kola M Owonikoko1 and Adetunji O Adeniji1
Received: August 09, 2024; Published: August 27, 2024
*Corresponding author: Oluseyi OA Atanda, Department of Obstetrics and Gynaecology, Osun State University Teaching Hospital, Osogbo, Nigeria
Wakeel O Muritala, Department of Obstetrics and Gynaecology, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria
DOI: 10.26717/BJSTR.2024.58.009147
Background: Malaria in pregnancy is associated with adverse maternal and foetal outcomes especially in sub-
Saharan Africa. Sulphadoxine Pyrimethamine (SP) is recommended for Intermittent Preventive Therapy (IPT)
against malaria in pregnancy. However, resistance to SP has been linked to reduction in its effectiveness. WHO
recommends uptake of SP at each Antenatal Care (ANC) visit at least four weeklies apart, but the effects of
number of doses of SP on clearance of malaria parasitaemia and pregnancy outcome still require comprehensive
review.
Objectives: To determine the prevalence of maternal, umbilical cord and placental malaria parasitaemia and to
determine the impact of number of doses of IPT-SP on the prevalence of parasitaemia.
Methodology: Prospective Cross-sectional Study. IPT-SPs were administered to participants in ANC under
directly observed therapy at least four weekly apart. During labour two millilitres of maternal, umbilical cord
and placenta blood were collected for diagnosis of malaria by microscopy.
Results: A total of 192 (91.43%) participants completed the study. The prevalence of maternal, placenta and
umbilical cord malaria parasitaemia were 35.0%, 34.4% and 32.3% respectively, there is added benefit of more
than three doses of IPT-SP on the occurrence of malaria parasitaemia demonstrating statistically significant dose
dependent relationship in all parity (p<0.001) and adverse drug reactions among the number of doses were not
statistically significant (p=0.395).
Conclusion: Four or more doses of IPT with SP were more effective in clearing malaria parasitaemia and
symptomatic malaria than 2-dose regimen and the extra dose of SP was well tolerated.
Keywords: Malaria; Sulphadoxine-Pyrimethamine; Intermittent Preventive Therapy; Parasitaemia; Placenta; Umbilical Cord; Lautech
Abbreviations: ANC: Antenatal Clinic; DHFR: Dihydrofolate Reductase; DHPS: dihydropteroate synthase; DHS: Demographic Health Surveys; DOT: Directly Observed Therapy; EDTA: Ethylene Diamine Tetra Acetic Acid; ERG: Evidence Research Group; HIV: Human Immunodeficiency Virus; HRP II: Histidine Rich Protein II; IPT: Intermittent Preventive Treatment; IPT: Intermittent Preventive Treatment; SP: Sulphadoxine-Pyrimethamine; IUGR: Intrauterine Growth Restriction; LAUTECH: Ladoke Akintola University of Technology; LBW: Low Birth Weight; LLIN: Long-Lasting Insecticidal Nets; LTH: Ladoke Akintola University of Technology Teaching Hospital; O&G: Obstetrics and Gynaecology; OGB: Ogbomoso; PCV: Packed Cell Volume; P. Falciparum: Plasmodium Falciparum; pLDH: Parasite Lactate Dehydrogenase; RBM: Roll Back Malaria; RDT: Rapid Diagnostics Test; SP: Sulphadoxine-Pyrimethamine; WHO: World Health Organization
Malaria remains a complex and overwhelming health problem[1]. Plasmodium falciparum malaria during pregnancy presents significant risks for the pregnant woman, [1] the developing fetus and the newborn infant [2,3]. The complications associated with malaria in pregnancy include severe malaria, severe anaemia, pre-term delivery, maternal death, and placental malaria [4-6]. Placental malaria is linked to intrauterine growth retardation, stillbirth, and delivery of low birth weight (LBW) infants [7,8]. An estimated [3.4] billion people are at risk of malaria[1]. The prevalence of malaria in pregnancy is estimated to be about twenty eight percent [4]. In 2012, there were an estimated 207 million cases of malaria and an estimated 627,000 deaths [1]. Each year, between 75,000 and 200,000 infant deaths are attributed to malaria infection in pregnancy globally [9,10]. Of the 125 million pregnant women who are at risk of Plasmodium falciparum infection each year, 30 million are from sub-Saharan African [11]. The disease is regarded as a major limitation to economic development in tropical and subtropical regions [12]. In Nigeria, the incidence of malaria -induced pregnancy death is about 11 per cent while malaria is a major contributory factor to anaemia during pregnancy with about 22 per cent mortality rate [13].
In addition to the direct impact of malaria, there are also several social and economic burdens on communities and the country as a whole with about 132 billion naira lost to malaria annually in form of treatment costs, prevention and economic loses [13,14]. In areas where malaria is highly endemic, a protective semi-immunity against P. falciparum is acquired during the first 10 to 15 years of life, and the majority of malaria-related morbidity and mortality occur in young children [15]. However, in contrast with low malaria prevalence in adults, pregnant women in endemic areas are highly susceptible to malaria, and both the frequency and the severity of disease are higher in pregnant women [16]. Pregnancies in women living in malaria endemic regions are associated with a high frequency and density of P. falciparum parasitaemia, [17,18] with high rates of maternal morbidity, including fever and severe anemia, miscarriage, and placenta malaria[17,18]. Given the health risks that malaria infection during pregnancy poses to the mother, her unborn fetus, and the newborn, adequate measures should be taken to treat and prevent malaria infection during pregnancy.3 IPT helps to reduce maternal malaria episodes, maternal and fetal anemia, maternal and placental parasitaemia, low birth weight, and neonatal mortality [19]. In spite of evidence of falciparum malaria resistance to SP its relevance in the intermittent preventive treatment of malaria in pregnancy (IPT) is reasonable by lack of a better alternative drug to serve the same purpose.
WHO revised the IPT policy recommendation in September 2012, increasing the regimen from at least two doses of SP to the provision of a dose of IPT-SP given at every ANC visit commencing as early as possible in the 2nd trimester throughout pregnancy and at least four weeks apart, administered as directly observed therapy. Appraising the impact of the policy review will require household surveys to include data on two, three, and more than three doses of IPT-SP. In sub-Saharan Africa, the effectiveness of IPT-SP in preventing malaria- related adverse pregnancy outcomes is well established [20-22]. In spite of the widespread adoption of IPT-SP in malaria endemic countries in Africa, the coverage of recommended doses of IPT-SP remains elusive [23]. In 2012, the median coverage of at least one, two and three doses of SP during pregnancy in sub-Saharan Africa was 64% (range 25-85%), 38% (range 10-64%), and 23% (range 2-44%), respectively [24]. The dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps quintuple mutant alleles have been strongly linked with SP treatment failure and has reached levels about 90% in parts of East Africa,[25] but lower in West Africa, <10% in Republic of Benin,[26] and 35.21% in Makurdi, Nigeria [27]. There are at present limited studies on similar subject in East Africa with most studies largely in East African countries, this study will add to the body of knowledge available on the subject in our environment.
It will provide valuable information on the effectiveness of IPT-SP in transmission settings that have low prevalence of dhfr/dhps mutant alleles. While previous studies have examined IPT-SP outcomes in areas of high prevalence of the quintuple SP mutations, sometimes demonstrating that protective effectiveness is waning or IPT-SP is harmful, however, in the West Africa region, there is lower prevalence of the quintuple mutation[26,27]and continued effectiveness of IPTSP is likely to remain high.
Aim
To determine the effect of number of doses of IPT-SP on maternal, umbilical cord and placenta malaria parasitaemia among parturient delivering at LTH, Ogbomoso
Specific Objectives
1. To determine the prevalence of maternal peripheral malaria
parasitaemia by microscopy (peripheral blood film).
2. To determine the prevalence of placenta malaria parasitaemia
by microscopy.
3. To determine the prevalence of umbilical cord malaria parasitaemia
by microscopy.
4. To identify the impact of number of doses of IPT-SP on the
prevalence of maternal, umbilical cord and placental malaria
parasitaemia.
5. To identify the impact of number of doses of IPT-SP on adverse
maternal and neonatal drug reaction.
It is a prospective cross-sectional study. The study was carried out at LAUTECH Teaching Hospital (LTH), Ogbomoso in Ogbomoso North Local Government area of Oyo state, South western, Nigeria. Malaria is highly endemic in this area with highest transmission occurring between May and October [28] and low incidence of disease in the dry season beginning from November to April [29]. The study was conducted among consented parturient who met the inclusion criteria presenting at booking in LTH, Ogbomoso. Inclusion criteria are: Singleton pregnancy, Gestational ages between < 32 weeks, Seronegative for HIV, Free of an acute illness requiring hospital admission, Willingness to deliver in the hospital. Pregnant women with prior adverse drug reaction to sulfa-containing medications, Anaemic patients, patients with Multiple gestation, chronic disease likely to influence pregnancy outcome e.g. Hbss and patients with prior major pregnancy complications e.g. pre-eclampsia were excluded from the study.
All consented participants were recruited at booking clinic using systematic sampling technique, they were duly educated about the study procedure at the antenatal educational sessions. Informed consent were obtained, Ultrasound scanning was requested for dating for those who are not sure of their last menstrual period or with no prior ultrasound scanning. Three tablets of SP containing 500mg sulphadoxine and 25mg pyrimethamine per tablet (Maldox® Emzor Pharmaceutical PLC, NAFDAC Reg. no; 04-2911) were administered under directly observed therapy at the ANC and recorded in the patients’ case notes.
Each patient was observed for thirty minutes after administration of SP for vomiting. One participant vomited within thirty minutes of administration and had the dose repeated. SP was administered every four weekly till delivery, at each follow-up visit, participants were asked about adverse drug reactions to SP, malaria symptoms, and routine obstetric issues, any symptomatic women found to be parasitaemic were given artemether-lumefantrine. Packed cell volume was checked btw 34-36 weeks of gestation. At delivery, ANC cards were checked for the number of doses of SP-IPT taken during pregnancy and the corresponding gestational age and such information recorded in the proforma. Two mls of Maternal venous blood, umbilical cord blood and placenta blood were collected for diagnosis of malaria by microscopy. Obtained data were analyzed using (SPSS) version 21. Participation is voluntary all information gathered was kept confidential. Participants were only identified by hospital number without their name. Data obtained from the study was stored in my personal passworded computer. Ethical clearance was obtained from the ethical committee of LTH Ogbomoso, PROTOCOL NUMBER: LTH/OGB/ EC/2017/144. Written informed consents were obtained from the Participants. Women found to have malaria at delivery were treated with anti-malarial.
A total of two hundred and ten (210) participants were recruited into the study between 5th February, 2018 till 24th august, 2018 (seven months) and were followed up. Sample collection at delivery was completed on 26th October 2018 (Nine months). Nine (4.3%) participants were lost to follow up, 4 (1.9%) were followed up till term but did not deliver in the hospital while in 5 (2.4%) participants placenta and cord samples were not collected but only maternal blood sample was collected. The median number of doses received by the study participants is three (3) doses, 92 (45.8%), 54 (26.9%), 33 (16.4%), 16 (8.0%) and 6 (3.0%) participants received 2, 3, 4, 5 and 6 doses respectively. (Table 1) showed the socio-demographic characteristics of study participants with regards to number of doses of IPT-SP taken in pregnancy. The age of participants ranges between eighteen (18) years and forty-three (43) years with mean age 29.62 ± 4.66, the lower the mean age the more likely the number of doses taken by the participants (ANOVA, F-2.647 sig-0.035, non-linear). One hundred and ninety-six (97.5%) participants were married while 5 (2.5%) were single. One hundred and eighty-three participants (91%) were Yoruba while 18 (9.0%) were Igbo, no other tribes found during the study. Seventy (34.8%), 17 (8.5%), 64 (31.8), 12 (6.0) and 38 (18.9%) participants were skilled, semi-skilled, un-skilled, housewife and undergraduate respectively. Majority (62.2%) of participants have more than secondary level of education.
Note: *Statistical significance p<0.05.
χ2 – Chi square test
LR – Likelihood ratio,
t – independent t-test
One hundred and thirty-two (65.7%) participants were Christian while 69 (34.4%) were Muslim. (Table 2) showed the gravidity and events in index pregnancy among the studied participants, the gestational age at booking of study participants ranges between 11 and 32 weeks, median is 22 weeks, the lower the mean gestational age the higher the number of doses taken by the study participants (F=9.473, p<0.001). The number of antenatal clinic visits of study participants ranges between 2 and 9, median is 5 visits, the higher the number of visits the higher the number of IPT-SP doses taken by the study participants (F- 107.162 p<0.001). Ninety (44.8%) participants sleep under insecticide treated net with no significance difference across the doses of IPT-SP taken (X2=3.054, p=0.549). The prevalence of maternal, placenta and umbilical cord malaria parasitaemia by microscopy are 35.0%, 34.4% and 32.3% respectively. (Table 3) Illustrate the efficacy of number of doses at preventing malaria parasitaemia, there is added benefit of more than 3 doses of IPT-SP on the clearance of maternal, placenta and umbilical cord malaria parasitaemia demonstrating statistically significant dose dependent relationship in all parity (p<0.001). As shown in the table, 48 (55.8%), 12(23.5%), 5 (15.2%),1 (6.3%), 0 (0.0%) participants who took 2, 3, 4, 5 and 6 doses of IPT-SP have placenta malaria parasitaemia respectively, which shows that with each number of doses, the prevalence (%) of placenta malaria parasitaemia is almost halved till total clearance at 6th dose.
Note: * Statistical significance p<0.05
LRχ2 – Likelihood ratio Chi square test
t – independent t-test
Table 3: Placenta, umbilical cord and maternal malaria parasitaemia vs. the number of doses of IPT-SP taken by the study participants.
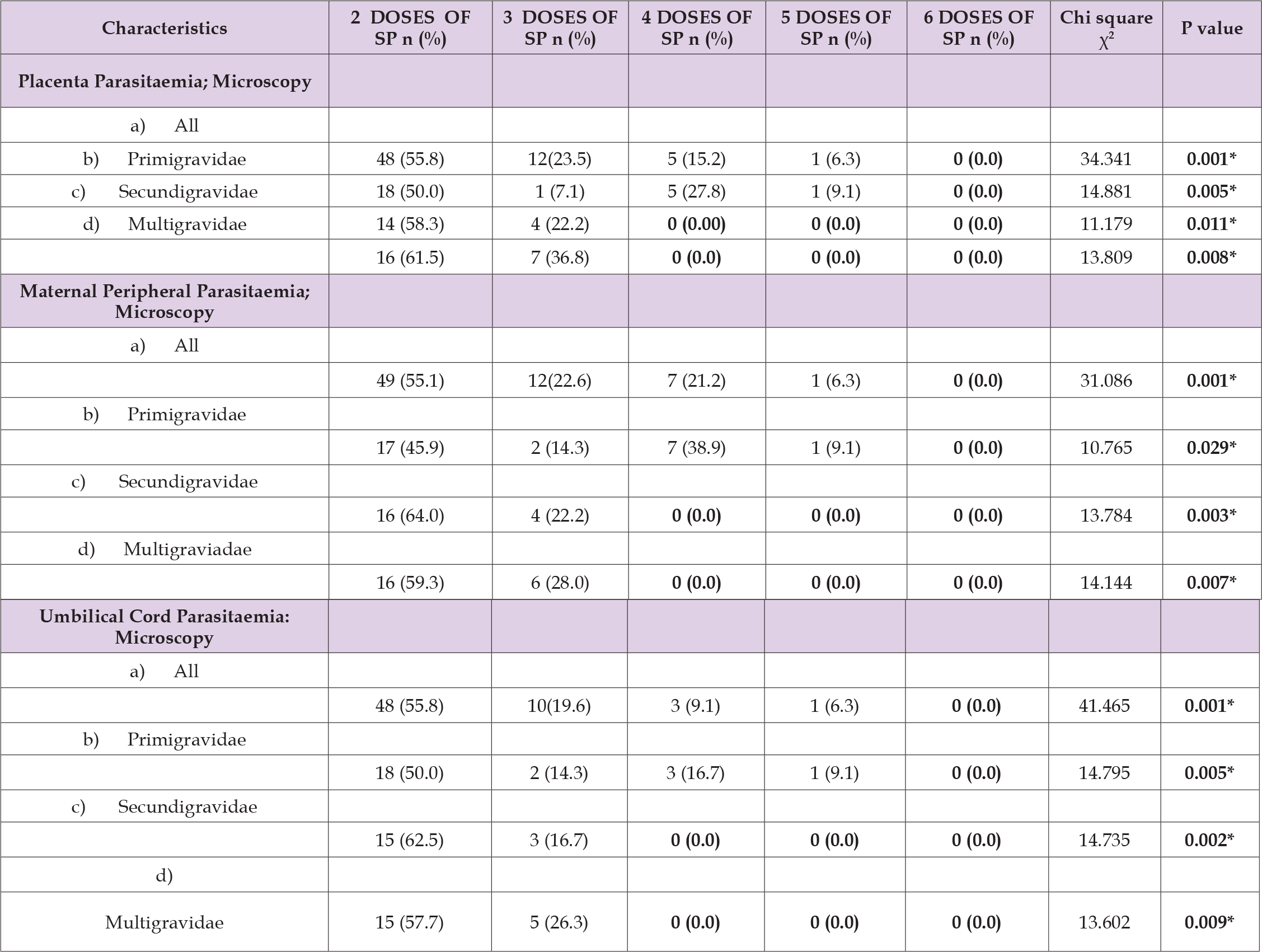
Note: * Statistical significance p<0.05
The threshold dose of IPT-SP for maternal, placenta and umbilical cord malaria parasitaemia clearance in secondigravidae and multigravidae is 4th dose with no added benefit of increasing doses but malaria parasite clearance in primigravidae is achieved at 6th dose. The uptake of SP-IPT within four weeks to delivery is significantly associated with lower risk of placenta (0.002), maternal (p = 0.006) and umbilical cord (0.001) malaria parasitaemia as shown in (Table 4). There is no participant with major adverse drug effect, only 15 (7.5%) participants have minor adverse drug reaction which include nausea 2 (1.0%), vomiting 4 (2.0%) and body weakness 9 (4.5%) as shown in (Figure 1) and in no case was SP withheld because of these concerns. There were no significant differences in the proportion of women reporting adverse drug reactions among the number of doses (χ2=4.085, p-value-0.395). Only one neonatal death (0.498%) was found, however, the cause of death is attributed to congenital anomaly (Edward syndrome), prematurity and very low birth weight. Only one case of neonatal hyperbilirubinaemia (0.5%) was seen in babies delivered by the participants, this was in a rhesus negative secundigravidae who took only 2 doses of IPT-SP with interval of last dose to delivery of 9 weeks.
Note: * Statistical significance p<0.05
χ2 – Chi square test
This study found that in Ogbomoso, South West, Nigeria, with high transmission rate of Malaria but low SP resistance, SP-IPT protected pregnant women from placenta, maternal and umbilical cord parasitaemia and poor birth outcomes. The degree and type of protection conferred by SP-IPT was dependent on the gravidity, and number of IPT-SP doses taken. This work provides valuable information on the continued effectiveness of IPT-SP in transmission settings that have low to moderate prevalence of dhps and dhfr mutant alleles [27]. Previous studies have examined IPT-SP outcomes in areas of high prevalence of the quintuple SP mutations, sometimes demonstrating that protective effectiveness is waning, or IPT-SP is harmful. Alternatively, in the West Africa region, while SP mutations are not uncommon, there is lower prevalence of the quintuple mutation27, and continued effectiveness of IPT-SP is likely to remain high. Similar age range was gotten by (Mace KE, et al. [30]) in Zambia with a age range of 16-44 years but the median age was 23 years [30]. There is no significant difference in the socio-demographic characteristics of study participants across the number of doses, this is contrary to what was found by (Mpogoro, et al. [31]) in Tanzania where the uptake of ≥3 doses of SP during pregnancy was significantly associated with higher level of education (OR = 4.22, 95% CI, 1.85-9.62, p = 0.001), and being employed or doing business (OR = 4.35, 95% CI, 1.85-10.10, p = 0.001),[31] the reason for the no significant difference may be due to regular emphasis of the important of regular ANC visits to participants and phone call when they missed their ANC visits.
The median number of doses received by the study participants is three (3) doses, more than half of the participants (54.2%) received ≥ 3 doses of IPT-SP, this uptake is more than what was found by previous studies; 33% by (Mace, et al. [30]) in Zambia,30 29.8% by (Azizi et. al [32]) and only 6.03% by (Mpogoro, et al. [31]) in Tanzania. 31 The increase in the uptake of IPT-SP could be related to frequent reminder and phone call of participants on regular ANC visits. The median gestational age at which participants received the first IPT-SP was 23 weeks (ranges from 16 to 32 weeks), this was greater than what was found by (Mpogoro, et al. [31]) whose median gestational age at which participants received the first dose of SP was 20 weeks (SD: 3.9) ranging from 16 to 34 weeks,[5] the reason may be related to delay in ANC booking in our environment. Despite IPT-SP prophylaxis the prevalence of placenta malaria was high 34.4%, this can be attributed, in part, to increased transmission intensity and low uptake of IPT-SP. This prevalence of placental malaria is similar to those reported in Cameroon (33.7%), Ghana (38.6%) and Tanzania 37.60% [31,33,34].but lower than the prevalence reported by (Ezebialu, et al. [33]) in Nnewi, Nigeria (63.3%)[35] and Aribodor, et al.in Awka, Nigeria (64.4%) [7] but was higher than previously reported by Mace, et al. in Mansa, Zambia, (4%), [30] in Uganda (17.5%) [36] and in Cameroon (25.5%) [37].
The prevalence of maternal peripheral malaria was 35.0% close to what was reported by Mpogoro FJ et. al in Tanzania 29.7% [31] but higher than what was reported by Mace, et al. in Mansa, Zambia [30]. The uptake of IPT-SP within four weeks to delivery is significantly associated with lower risk of Placenta, Maternal and Umbilical cord malaria parasitaemia. Similar findings was found by Arinatwe E, et al. where participants who reported taking SP 3–14 days before delivery had a significantly lower risk of a positive placental blood smear (2.8% vs. 17.8%, p = 0.02) and maternal peripheral parasitaemia (2.8% vs. 19.6%, p= 0.01) compared to those who reported taking their last dose of SP more than 14 days prior to delivery [36]. But Mace et al only found significant finding with maternal parasitaemia (8.7% vs. 16.8%, p=0.02) but no effect of timing of last uptake of SP on placenta infection [30]. There is good effect of more than 3 doses of IPT-SP on the occurrence of maternal, placenta and umbilical cord malaria parasitaemia demonstrating statistically significant dose dependent effect in all parity (p<0.001) with each number of doses the prevalence of placenta malaria parasitaemia is almost halved till total clearance at 6th dose. Similar finding was found by Maiga et al in Mali where at delivery, the prevalence of placental malaria in the 3-dose group was half that in the 2-dose group (8.0% vs. 16.7%; APR, 0.48; 95% CI, 0.32–0.71) [20]. Gutman J, et al. in Malawi, also noted a non-significant but dose-dependent relationship between placental infection and number of SP doses [38]. However, among primigravidae, there was a clear dose-dependent effect, with a significant effect of each dose increase [38].
This supports the WHO recommendation of monthly dosing of SP for IPT, even in areas of low to moderate resistance to SP. However, the threshold dose of IPT-SP for maternal, placenta and umbilical cord malaria parasitaemia clearance in secondigravidae and multigravidae is 4th dose with no added benefit of increasing doses but malaria parasite clearance in primigravidae still demonstrate dose dependent benefit up till 6th dose. In the findings of Filler SJ, et al. in Malawi [39] and (Arinaitwe E, et al. [36]) in Uganda, 36 women who took monthly SP were less likely to have placental malaria than women who took 2-dose SP, but the difference did not reach statistical significance (RR, 0.37 [95% CI, 0.11–1.19]) [39] and (OR = 0.75, p = 0.25) [36] respectively. The study by Mpogoro FJ, et al. in Tanzania, in multivariable analysis, adjusted for age, gravidity, bed net use and ethnicity; [31] women who took three or more doses of IPT-SP had a lower odd of placental parasitaemia when compared with those who took less than 3 doses (AOR = 0.31, p = 0.039) [31]. However, receipt of ≥ three doses of IPT-SP was not associated with reduced odds of peripheral malaria [28]. The lack of association could be explained by the fact that a small proportion of women in the study took the recommended three or more doses of SP during pregnancy [31].
However, Hamer DH, et al. in Zambia, found no evidence that intensive monthly dosing was superior to two doses of IPT-SP in HIV-seropositive women in terms of placental malaria, maternal anemia, or birth outcomes [40]. Although rates of placental parasitaemia, maternal peripheral parasitaemia, symptomatic malaria episodes and cord blood parasitaemia were lower in the monthly dosing group, none of these differences were statistically significant and the absolute reduction for each parameter was relatively small [40]. There is no participant with major adverse drug effect, no severe cutaneous ADR was also found by (Maiga, et al. [20,39]) In the study of Hamer DH, et al. in Zambia the rates of serious adverse events were low and did not differ between the study groups for infants (RR for I-IPTp vs. S-IPTp, 1.49 [95% CI, 0.79 –2.8]) or mothers (RR for I-IPTp vs. S-IPTp, 1.13 [95% CI, 0.56 –2.18]) [40]. Only 15 (7.5%) participants have minor adverse drug reaction which include nausea 2 (0.995%), vomiting 4 (1.99%) and body weakness 9 (4.48%), this is similar to the finding by Filler SJ et. al in which only 1% of women reported adverse drug reactions. There were no significant differences in the number of women reporting adverse drug reactions among the number of doses (X2=4.085, p=0.395), this is similar to previous findings [20,39,40]. The paucity of adverse drug reactions associated with SP and the lack of association with adverse maternal or fetal outcomes are also consistent with the findings of other published trials [20, 39 & 40].
1. IPT-SP showed a dose-dependent effect on placenta, umbilical
cord and maternal malaria parasitaemic clearance.
2. Placenta, umbilical cord and maternal malaria parasitaemic
clearance is achieved at 4th dose in secundigravidae and
multigravidae and at 6th dose in primigravidae
3. Low rate of adverse drug reactions and the adverse drug reactions
are not related to increasing doses of IPT-SP
4. The uptake of IPT-SP within four weeks to delivery is associated
with clearance of malaria parasitaemia
Pregnant women should be encouraged to receive four or more doses of IPT-SP in pregnancy and Uptake of IPT-SP within four weeks to delivery should be encouraged.
Profound gratitude to the then Head of Department of Obstetrics and Gynaecology; Prof. A.S Adeyemi and the entire consultant staff of the Department of Obstetrics and Gynaecology of LAUTECH Teaching Hospital, Ogbomoso for their guidance and for allowing their patients to participate in this research. Appreciation also goes to Dr. A.A Akintunde and Dr. Olufemi Aworinde for their meticulous correction of the dissertation. My appreciation go to all research assistants, residents, nursing staff and other staffs in the department of Obstetrics and Gynaecology, LAUTECH Teaching Hospital, Ogbomoso for their useful advice, help and support while carrying out the research, you are all highly appreciated. My gratitude also goes to the management of LAUTECH Teaching Hospital, Ogbomoso for providing the enabling environment to carry out this study. I want to sincerely acknowledge especially all participants who took part in this study.
The authors declared that they have no conflict interest.


