Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Töysä T*
Received: July 18, 2024; Published: August 23, 2024
*Corresponding author: Töysa T, Licentiate of Medicine, Specialty General Practice, Retired, Student of Eastern Finland, Kuopio, Finland
DOI: 10.26717/BJSTR.2024.58.009142
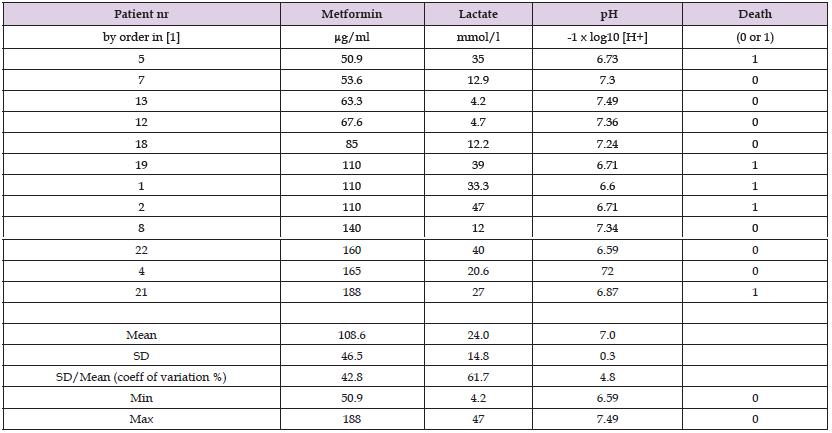
This article is based on the data collected and published by Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD and Morgan BW in 2009 [1]. They have been even analyzed in [2] with regression analyses, without graphics and without clear conclusions. Table 1 presents a subpopulation from [1] with S-metformin (Met), S-lactate (Lact) and serum nadir (lowest detected) pH (pH) values and outcomes.
Cases were selected by arranging patients by their increasing Met values including all (5) non-survivors. Minimum Met was 50.9 µg/ml (= mg/l). Survivors with lower Met value than 50.9 mg/l were not included. Total number of cases was 12. The patients are labeled by their data row in the original Table 1 in [1]). Table 1 includes even means, standard deviations (SD), coefficients of variation, minimal and maximal values of each parameter. Additional units (y/max) has been used for Lact and Met, respectively, in order to enable efficiently the use of three parameters per table. In all figures Met is remarked by the same line-type. Death is remarked by black columns, exceptionally surviving (patient nr. 22) with a white column, in order to be detected, especially in Figure 3. Original data are analyzed with IBM SPSS Statistics, version 29 and MS Excel as in [2].
Table 1: Patients with metformin overdose and their serum Met, Lact and pH values and Outcomes of their treatments (Death by survivors 0, by non-survivors 1).
As a single parameter Met explained mortality by 1 %, non-significantly. Single Lact and pH explained death significantly (p < 0.01) by more than 50 %. Combined regression by Met, Lact and pH explained mortality by 59 %, p = 0.059 (nearly significantly) (Table 2).
Strongest factor by beta coefficients was pH. Influence of Met was negative (anti-lethal). Anyhow the statistical power of the of the beta coefficients was very low. Maybe the most important observations are the high Met values (mean 109, min 51 mg/l, which is 10-20 times higher to the reference values [3,4]) with 7 survivors and that the ratio of non-survivors/survivors was 1/3 in the lowest and in the highest one third of Met group. Single Lact nor pH could not explain significantly Met variation and vice versa (calculations are not shown here).
Generally high Lact (Figure 2) and low pH (Figure 3) are associated with lethality. Anyhow association between Met and Lact is not statistically significant neither causal [2] and could be explained in several cases by magnesium and ATP deficiency [2]. In accordance with [2] higher ingested metformin dose is reported to have been associated with a better outcome in ICU patients with metformin-associated hyperlactatemia/LA [5]. Experimentally S-Lact increase has been produced by dietary Mg deficiency [6,7]. Irritable swine, with increased muscle tonus and sterile fever, obviously sows (estimated weight 200 kg [8]) with supposed hyperlactatemia have been treated successfully with 4 g Mg (100 ml solution with 20 % of MgSO4) subcutaneously [7] and results were seen in one hour. Magnesium narcosis is known in old textbooks [9] and Mg anesthesia is well known in scientific world [10]), but obviously not in everyday practice. It is clear that the upper (fasting) S-Mg reference value of humans (0.94 mmol/l [11]) is become to be exceeded with respective [7] (1.3 g = 55 mmol) Mg treatment [= 4g x (70 kg/200 kg)], although given subcutaneously, i.v. infusions (slow) should be monitored by clinical symptoms (including ECG), because tissue Mg is decisive. Remarkable is that the upper human reference of S-Mg is below the range of the pig reference S-Mg values (0.97 – 1.45 mmol/l) [12], which suggests on the role of Mg in human lactic acidosis. Monitoring of body Mg via bone (trochanter) or muscle biopsies [2,13,14] (sparcely) [13] are suggested.
Magnesium supplementation is suggested for the treatment of lactic acidosis under the guidance of anesthesiologist before haemodialysis. Monitoring of body Mg via muscle and bone biopsies can be beneficial.
I am grateful to Professor Osmo Hänninen and veterinary surgeon Seppo Haaranen for several discussions.


