Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Michael AS Guth*
Received: July 25, 2024; Published: August 14, 2024
*Corresponding author: Michael AS Guth, (mike@michaelguth.com) www.linkedin.com/in/populationhealthmanagement
DOI: 10.26717/BJSTR.2024.58.009118
The CARES-310 clinical trial compared the safety and efficacy of camrelizumab, an anti-PD-1 antibody, combined with rivoceranib, a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted tyrosine kinase inhibitor (TKI), versus the obsolete standard of care sorafenib as first-line (1L) treatment for unresectable hepatocellular carcinoma (uHCC). In this independent analysis of the CARES-310 trial, potential bias in the choice of comparator and the clinical significance of the results are reexamined. Although rivoceranib (also known as “apatinib”) is approved in China to treat patients with advanced gastric cancer, the clinical trial conducted in the USA for patients with gastric cancer seems to indicate missed endpoints and weak evidence to justify FDA approval. Due to a nondisclosure agreement, the principal investigators are unable to comment on these results. However, from a table of functional benefits and harms, readers might well conclude that palliative hospice care is non-inferior to rivoceranib for patients with Stage 4 gastric cancer.
Independent analyses of drug clinical trials, i.e., analyses not sponsored or paid for indirectly by pharmaceutical manufacturers, are needed in medical literature [1-3].
“(Many reports indicate that medical journal articles are researched and written by or on behalf of pharmaceutical companies, and then published under the name of academics who had played little role earlier in the research and writing process.) In extreme cases, drug companies pay for trials by contract research organizations (CROs), analyze the data in-house, have professionals write manuscripts, ask academics to serve as authors of those manuscripts, and pay communication companies to shepherd them through publication in the best journals. The resulting articles affect the conclusions found in the medical literature and are used in promoting drugs to doctors.” [4] (internal citations omitted).
A second criticism of published drug clinical trials concerns methodological bias, in general, and the selection of a comparator, [5] in particular; because “the choice of comparator directly affects the validity of study results, clinical interpretations, and implications” [6]. This article reexamines the CARES-310 clinical trial of camrelizu- mab-rivoceranib (C+R) for treatment of uHCC with a focus on the choice of comparator, the clinical significance of the results, the sponsor’s recent history of clinical development and regulatory affairs, and a recent clinical trial in the USA for patient with advanced gastric patients with rivoceranib plus hospice care vs. hospice care alone.
CARES-310 compared the safety and efficacy of camrelizumab, an anti-PD-1 antibody, combined with rivoceranib (also known as apatinib), a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted tyrosine kinase inhibitor (TKI), versus the “outdated” single drug standard of care sorafenib as first-line (1L) treatment of unresectable hepatocellular carcinoma (uHCC) [7]. Sorafenib is an oral multi-kinase inhibitor and that blocks tumor angiogenesis through inhibition of VEGF. In prior clinical trials, PD-1 immune checkpoint inhibitors (ICI) combined with an angiogenic antagonist were found to increase overall survival (OS) in patients with renal cell carcinoma and HCC versus anti-angiogenic therapy alone [8]. The results from CARES-310 demonstrated statistically significant benefits in both progression-free survival (PFS) and OS in 1L treatment of patients with uHCC using the C+R combination therapy over sorafenib monotherapy.
Patients with uHCC in the CARES-310 study were randomly assigned to one of two treatment groups [9]. The first group received treatment with of camrelizumab 200 mg IV Q2W + rivoceranib 250 mg p.o. QD, continuously, 4 weeks (28 days) per cycle of therapy. The second group received sorafenib 400 mg p.o. BID 4 weeks (28 days) per cycle of therapy. The co-primary endpoints of the study were OS and PFS. In the published interim analysis comparing the C+R group to the sorafenib group, the median OS (mOS) was 22.1 months (95% CI, 19.1–27.2) versus 15.2 months (95% CI, 13.0–18.5), with Hazard Ratio (HR) 0.62 (95% CI, 0.49–0.80; p<0.0001). The median PFS was 5.6 months (95% CI, 5.5–6.3) versus 3.7 months (95% CI, 2.8–3.7) with HR 0.52 (95% CI, 0.41–0.65; p<0.0001) [9].
Treatment-related adverse events of grade 3 or higher occurred in 220 (81%) patients treated with C+R [9]. Dose reductions were necessary in 128 (47%) patients and permanent discontinuations of at least one medication in 66 (24%), showing that C+R therapy was associated with a higher risk of toxicity compared with sorafenib. Similarly, 44 (16.2%) patients in the C+R group required systemically administered corticosteroids to manage immune-related adverse events (irAEs) [9].
The Protocol for CARES-310 was amended to clarify the use of corticosteroids for the management of irAEs [10]. “(If the camrelizumab is delayed for 3-5 days and AST or ALT levels worsen, corticosteroids such as methylprednisolone 0.5-2 mg/kg/day or equivalent oral drugs will be administered.) In case of AST or ALT >8 times of ULN, cortisol, i.e., methylprednisolone 1-2 mg/kg/day or equivalent oral drugs, will be given immediately, and meanwhile, a consultation from the department of gastroenterology is advised. If AST or ALT levels worsen 3-5 days after the start of corticosteroids treatment, other immunosuppressants may need to be added, such as mycophenolate 1g BID” [10]. Table 1 shows the most frequently occurring irAEs including reactive cutaneous capillary endothelial proliferation (RCCEP) in the CARES-310 clinical trial. No grade 5 adverse event occurred, and RCCEP was one of the most frequently occurring irAEs. [10] The innocuous statement that 44 (16.2%) patients required systematically administered corticosteroids means these seriously ill patients suffered such intolerable adverse events that they were then subjected to suppression of their remaining immune system function and thereby increased risk for infection and cancer metastases post C+R therapy.
Table 1 shows that at the interim analysis point, apparently Feb. 8, 2022, in the C+R group (n=272), 72 (26%) patients had Grade 1-2 RCCEP, and 7 (3%) patients had Grade 3 RCCEP. Thus, in the CARES-310 study, just under 30% of the patients that received camrelizumab developed RCCEP at the interim analysis point in the follow-up period. None of the C+R group had Grade 4 or Grade 5 RCCEP, and no patients in the Sorafenib group (n=269) had RCCEP at any grade [9]. As shown in Figure 1 below, RCCEP can cause red-nevus-like, pearl-like, mulberry-like, patch-like, and even tumor-like skin lesions in patients with HCC. Furthermore, if the follow-up period had been longer, more patients in the C+R group may have suffered RCCEP adverse events than the n (%) shown in Table 1 consistent with the higher rates of RCCEP side effects reported elsewhere for patients with HCC treated with camrelizumab.
Table 1: CARES-310 C+R Group Immune-Related Adverse Events at the Interim Analysis for Overall Survival (Safety Analysis Set).
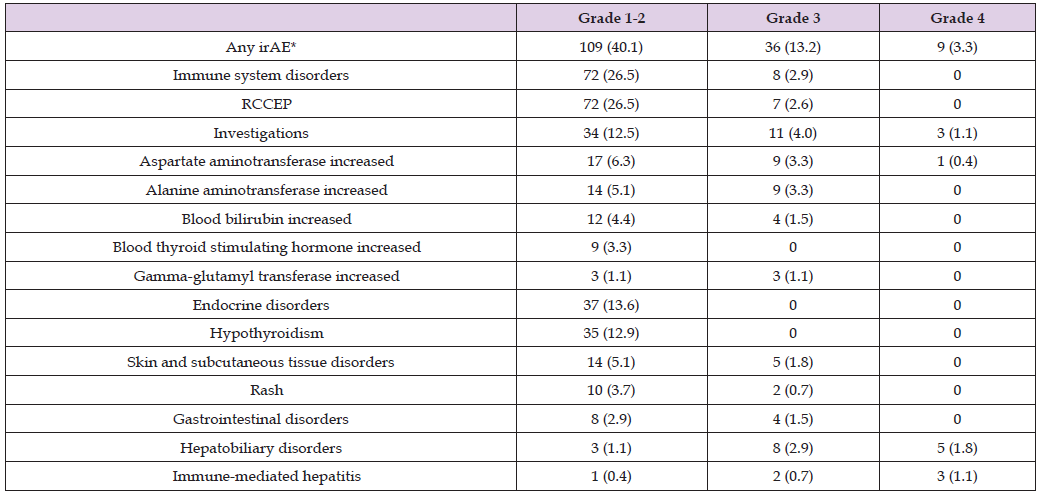
Note: Data cutoff was Feb. 8, 2022. Data are n (%). irAEs occurring in ≥2% of patients in the C+R group are listed. No grade 5 event occurred. *Systemically administered corticosteroids were required by 44 (16.2%) patients. irAE=immune-related adverse event; RCCEP=reactive cutaneous capillary endothelial proliferation. Source: [10], Table S13.
RCCEP, first identified by that name in [11] and previously known as reactive capillary hermangioma, is a burdensome irAE that occurs in the range of 66% - 88% of patients treated with camrelizumab [11,12]. No global consensus exists for the optimal treatment of RCCEP, which although not life-threatening, can affect work performance, social stigma, and low quality of life [12]. These skin lesions are particularly striking when they appear on the patients’ face and neck and may bleed. See the images displayed when searching the Internet using the keywords “reactive cutaneous capillary endothelial proliferation.” The CARES-310 Supplement identifies irAEs as one set of primary safety endpoints, [10] and given the growing number of immune checkpoint inhibitor – tyrosine kinase inhibitor (ICI-TKI) drug combinations approved by the FDA for treatment of metastatic carcinomas, oncologists and patients need to balance a potential increase in OS with enhanced risk of toxicity or decline in the quality of life. Expressed in more simple terms, patients undergoing cancer treatment may decline a therapy that poses risk of likely toxic side effects (81% of C+R group experienced adverse events of grade 3 or higher) [7].
In a comment on the CARES-310 clinical trial results published
in the prestigious medical journal, The Lancet, a group of academic
researcher-oncologists raised these points:
1. 450 (83%) of the 543 patients enrolled in the CARES-310
trial were from a single country outside of the USA and Europe,
and thus the clinical trial population wasn’t as demographically
stratified as the patient population for HCC [7].
2. That fact led to more than 85% of the clinical trial participants
having viral-related chronic liver disease, primarily secondary
to hepatitis B virus (>70%) [7].
3. “In endemic regions such as Asia, hepatitis B virus carriers
are often younger at hepatocellular carcinoma diagnosis with
consequently lower comorbid burden and lower prevalence of
cirrhosis compared to other causes of chronic liver disease. Furthermore,
when these hepatitis B virus carriers are on highly effective
antiviral treatments, they are often able to preserve liver
function” [7].
4. Non-alcoholic fatty liver disease (NAFLD), also known as hepatic
steatosis and steatotic liver disease, is emerging as the leading
risk factor for HCC in countries outside of Asia, [7] particularly
in the USA and Europe that follow the Western diet.
5. Few, if any, pharmacological treatments exist to prevent the
progression of NAFLD [7].
6. The “uneven representation of the full spectrum of the disease”
should be noted when considering whether results of the
CARES-310 study apply to wider uHCC patient populations [7] ,
such as those in the USA and Europe.
7. “Geographical heterogeneity in the standard of care” for HCC as well as real-world evidence on “etiology-specific therapeutic vulnerabilities in viral vs. non-viral HCC” will continue to create controversies in the optimal drug combination to treat uHCC [7]. Sorafenib is an “outdated” [7] standard of care for HCC. Better comparators, such as atezolizumab-bevacizumab or durvalumab- tremelimumab, [7] were available to compare for safety and efficacy with the proposed C+R therapy.
8. Compared to sorafenib, C+R therapy was associated with a higher risk of toxicity and intolerable side effects leading to reductions in dosing and cessation of at least one medication [7].
9. “In CARES-310, estimates can at the time of study publication be confidently reconstructed for global health status only, and these showed a worse median time to deterioration for C+R (median 11.2 months, 95% CI 7.6-not reported [NR]) compared with sorafenib (median not reached, 95% CI 7.4-NR). Conversely, follow-up duration was not mature enough to quantify the median time to deterioration in physical function and role functioning in both groups. Taken together, these results show us that the cost of synergistic efficacy from ICI-TKI combinations might reside in a potential treatment-related deterioration in quality of life” [7].
The CARES-310 study appears to have enrolled numerous Chinese patients who may previously been exposed to hepatitis B or other viruses and were treated with antiviral drugs that prevented loss of liver function and who received their HCC diagnoses at a younger age and overall healthier condition than comparable patients diagnosed with HCC in the United States and Europe. A clinical trial patient population with younger age initial HCC diagnoses and fewer comorbid conditions may have been an unintended consequence of including such a high percentage of Asian/Chinese patients. Thus, the supposedly superior OS results for C+R therapy shown in CARES-310 may be attributed at least in part to an overrepresentation of younger patients with fewer comorbidity confounding factors than HCC patients in other parts of the world. Aside from its potential bias in enrolling younger patients and those with comparatively fewer comorbidities at time of HCC diagnosis, the CARES-310 clinical trial may have underrepresented patients with NAFLD, who are more widely reflected in the general population. Two known causes for NAFLD are diabetes and obesity, which are growing population health crises in various parts of the world, but particularly the USA and Europe.
In addition to the monoclonal antibody comparators suggested by the Comment authors above, the CARES-310 sponsors would have performed more useful medical research if they had compared C+R therapy to Roche’s combination therapy tecentriq-avastin or AstraZeneca’s combination therapy imjudo-imfinzi, both of which are FDA-approved for treatment of uHCC. In any dual arm clinical trial, the choice of the comparator typically draws immediate focus and scrutiny from outside reviewers. The OS comparative results might not have been as impressive for C+R if instead two-drug combination comparators had been selected.
The CARES-310 study was published as an interim analysis with the C+R median OS was 22.1 months versus 15.2 months for sorafenib. In a subsequent conference presentation, the CARES-310 Study Group revealed the final analysis of the CARES-310 data indicated the C+R median OS was 23.8 months versus 15.2 months for sorafenib [13]. “OS benefits with C+R were generally consistent across subgroups, regardless of geographical region, race, and etiology” [13]. A reformulated clinical trial with a leading therapy as comparator might have added new insights into the relative toxicity and quality-of-life factors of marketed (in the case of the Roche and AstraZeneca drugs) and a proposed (C+R) uHCC treatment. Oncologists and patients with uHCC must weigh the relative benefit of survival against the increase in toxic side effects of any treatment. Although the CARES-310 data revealed a statistically significant PFS over sorafenib, those results might have little clinical importance [7] given the prognosis for patients with metastatic HCC or uHCC. Simply put, is the proposed therapy merely extending the patient’s misery or giving the patient a (temporary) improvement in quality of life?
Elevar Therapeutics, which is an American subsidiary of a Korean holding company, sponsored the CARES-310 trial but has had a chaotic clinical development and regulatory affairs history in the past few years. The U.S. Department of Health and Human Services’ ClinicalTrials. gov Internet site lists the following 9 clinical trials associated with Elevar Therapeutics.
COMPLETED
NCT05287360
A Study Comparing Four Different Rivoceranib Tablets in Healthy
Participants
Conditions
Healthy
Locations
Tempe, Arizona, United States
TERMINATED
NCT04073615
A Study of Rivoceranib and Trifluridine/Tipiracil for Metastatic
Colorectal Cancer (mCRC)
Conditions
Metastatic Colorectal Cancer
Locations
Sarasota, Florida, United States
Chicago, Illinois, United States
Saint Louis, Missouri, United States
Nashville, Tennessee, United States
Reason for Termination: Phase 2 portion of the study was not performed due to redirection of the rivoceranib development plan by the Sponsor.
TERMINATED
NCT04119453
A Study to Evaluate the Efficacy and Safety of Rivoceranib in Participants
With Recurrent or Metastatic Adenoid Cystic Carcinoma
(ACC)
Conditions
Adenoid Cystic Carcinoma
Locations
Los Angeles, California, United States
San Francisco, California, United States
Denver, Colorado, United States
Tampa, Florida, United States
Show 11 more locations
Reason for Termination: The RM-202 study was terminated by the
Sponsor due to redirection of the rivoceranib development plan.
TERMINATED
NCT03707028
A Study to Evaluate Safety, Tolerability, and Efficacy Profile of
Rivoceranib With Paclitaxel in Advanced Gastric or Gastroesophageal
Junction Cancer
Conditions
Gastric Cancer
Gastroesophageal Junction Adenocarcinoma
Locations
Seoul, Korea, Republic of
Reason for Termination: Terminated after completion of Part 1
due to a change in research plan.
COMPLETED
NCT03396211
A Study to Evaluate Apatinib (Also Known as Rivoceranib) Plus
Nivolumab in Participants With Unresectable or Metastatic Cancer
Conditions
Cancer
Locations
Santa Monica, California, United States
COMPLETEDWITH RESULTS
NCT03042611
A Study to Evaluate Rivoceranib Plus Best Supportive Care Compared
to Placebo Plus Best Supportive Care in Participants With
Gastric Cancer
Conditions
Gastric Adenocarcinoma
Gastric Cancer
Locations
Scottsdale, Arizona, United States
Rogers, Arkansas, United States
Detroit, Michigan, United States
Rochester, Minnesota, United States
Show 95 more locations
COMPLETED
NCT01497704
Dose-Escalation and Safety Trial of YN968D1
Conditions
Cancer Patients with Solid Tumors
Locations
Salt Lake City, Utah, United States
Seoul, Korea, Republic of Korea
NOT YET RECRUITING
NCT06308575
NEW
A Phase II Study of Rivoceranib for Patients With Recurrent or
Metastatic Olfactory Neuroblastoma
Conditions
Metastatic Olfactory Neuroblastoma
Recurrent Olfactory Neuroblastoma
Locations
Houston, Texas, United States
TERMINATED
NCT03407976
Apatinib With Pembrolizumab in Previously Treated Advanced
Malignancies
Conditions
Advanced Malignancies
Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
MSI-H or dMMR Solid Tumors
Urothelial Carcinoma
Locations
Salt Lake City, Utah, United States
One of the clinical trials listed above has yet to begin enrolling
patients. Nevertheless, four out of the remaining eight, or 50%, of
the clinical trials were terminated abruptly. The reasons given for
the clinical trial terminations suggest the clinical development plans
were half-baked when the clinical trials commenced and after the
parent company’s funds were spent. The time for changing a research
plan or drug development program is before launching the clinical trial
and enrolling patients. Large multi-site clinical trials are expensive,
and if publicly traded pharmaceutical firms abruptly terminated 50%
of their clinical trials, the firms’ shareholders would likely revolt and
oust senior management. Camrelizumab plus apatinib (rivoceranib)
combination therapy is approved in China as 1L treatment for uHCC
[9]; hence, the CARES-310 clinical trial sought to produce evidence
to support FDA approval of C+R as treatment for uHCC in the United
States. Similarly, since 2016 apatinib has been approved in China for
third-line (3L) or later-line treatment for advanced or metastatic gastric
or gastroesophageal adenocarcinoma [14].
Thus, clinical trial NCT03707028 captioned “A Study to Evaluate Safety, Tolerability, and Efficacy Profile of Rivoceranib with Paclitaxel in Advanced Gastric or Gastroesophageal Junction Cancer,” contained the same indication for advanced gastric or gastroesophageal adenocarcinoma but for rivoceranib paired with a traditional chemotherapy, paclitaxel. Nevertheless, a clinical trial conducted solely in South Korea might not support FDA approval in the United States. The reason for premature termination of the clinical trial, change in research plan, may be a euphemism for failed efficacy or safety endpoints. Clinical trial NCT03042611, “A Study to Evaluate Rivoceranib Plus Best Supportive Care Compared to Placebo Plus Best Supportive Care in Participants with Gastric Cancer,” for medical conditions gastric adenocarcinoma and gastric cancer, posted the following results as Table 2. These results show that for gastric cancer patients nearing the end of their lives and receiving hospice care, rivoceranib + hospice care has mixed results for functionality and harms when compared to hospice care alone. For some measures of functionality and adverse events, the patients receiving hospice care alone did better than the group that received rivoceranib along with hospice care. These results do not illustrate a clear clinical justification for adding rivoceranib when patients have no alternative but hospice care.
Table 2: Number of Participants Per QOL Dimension Response as Measured by the EuroQol 5-Dimension 5-Level (EQ-5D-5L) Questionnaire Type: Secondary | Time Frame: EOT (Month 24).
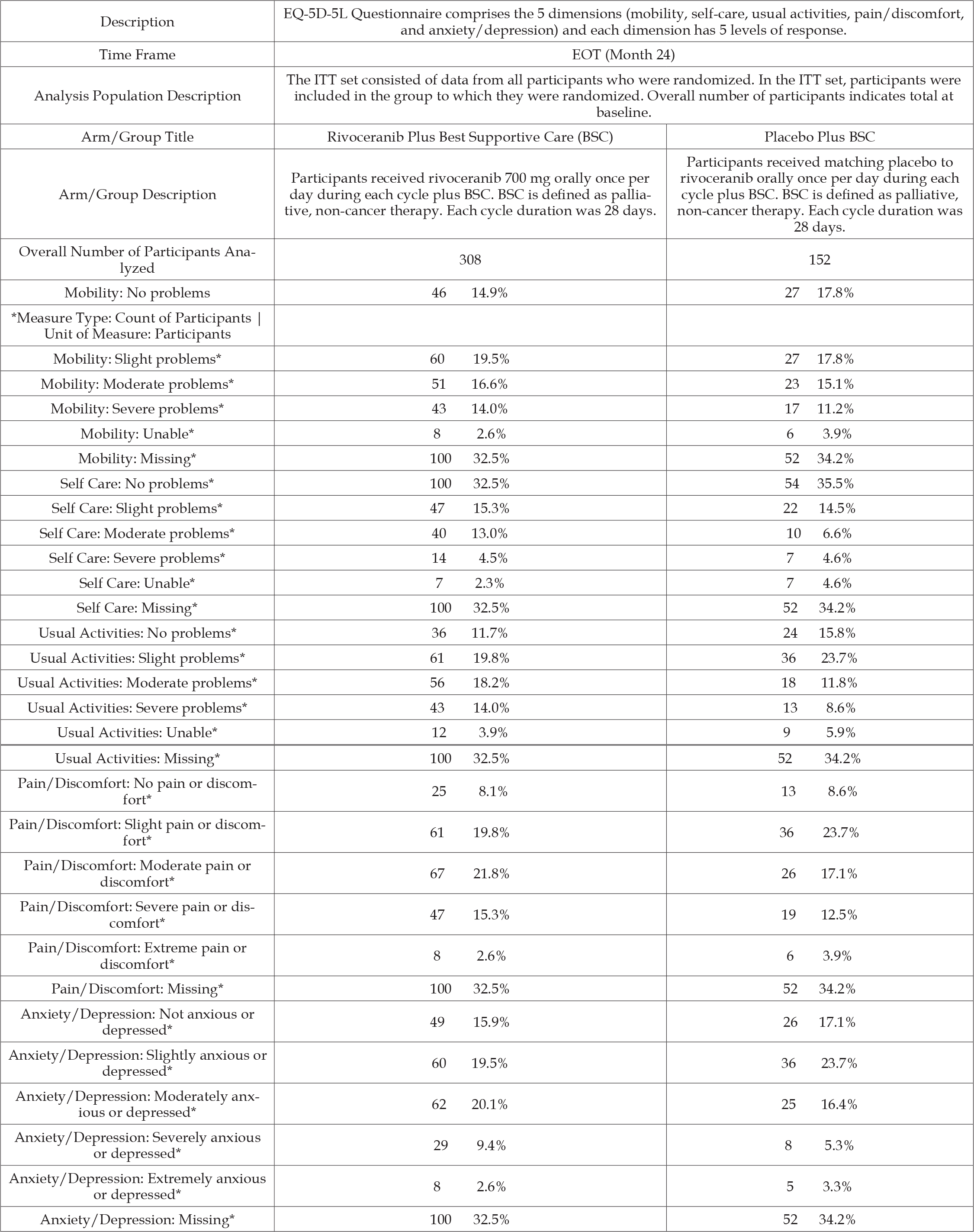
Note: *Measure Type: Count of Participants | Unit of Measure: Participants
From Table 1 of the reported results, the median OS for the rivoceranib- treated group was 5.82 months (95% CI 5.26 – 6.47) and for hospice care alone 5.13 months (95% CI 4.47-6.24). The median PFS disclosed in Table 2 of the reported results are 2.83 months (95% CI 2.07-3.52) for patients treated with rivoceranib and 1.77 months (95% CI 1.71-1.84) for hospice care alone. This dubious evidence is unlikely to support FDA approval for rivoceranib to treat gastric cancer in patients entering hospice care. Ominously, clinical trial NCT03042611 contains the following notice: “There IS an agreement between Principal Investigators and the Sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial is completed.” Thus, none of the academic oncologist researchers who are principal investigators for clinical trial NCT03042611 can publish or present at conferences the trial’s results. It is left to independent researchers to find, analyze, and present such clinical trial data with non-disclosure agreements binding the principal investigators against any comment.
In the past couple of years, Elevar Therapeutics eliminated a large percentage, perhaps as high as 33% of its staff. After one such layoff, Elevar’s Chief People Officer posted the following message on LinkedIn. com: “It was a tough few days for everyone at Elevar. With the unanticipated delay of an approval for our first New Drug Application, we’re focused on resubmitting to the FDA instead of launching a new cancer therapy for liver patients.” At the conclusion of the CARES-310 clinical trial, Elevar staff were jubilant and expected rapid FDA approval of C+R for uHCC and a new product launch for C+R. Instead, news service Fierce Pharma reported on May 17, 2024, “FDA snubs another China-made PD-1 with rejection of Elevar, Hengrui’s liver cancer combo” [15]. The article noted that the FDA accepted the clinical data of the CARES-310 trial, despite the valid criticisms raised by the Comment [7] to the trial, but “Hengrui has yet to resolve manufacturing deficiencies the FDA spotted during an inspection for camrelizumab, which has been approved in China under the brand name AiRuiKa for various indications” [15].
Elevar was caught off-guard. “The Complete Response Letter came as a surprise to Elevar because the company has been in label discussions with the FDA as recently as the past week, the company’s CEO Saeho Chong said in a statement to Fierce Pharma” [15]. The purpose of a pharmaceutical firm employing a Chief Regulatory Affairs executive and its staff is to maintain continual communication with the FDA and other regulators, anticipate FDA concerns and implement corrective measures, and advise the executive management team of any barriers to approval. Elevar Therapeutics had ample notice that the FDA inspections of pharmaceutical manufacturing facilities outside of the USA, particularly in China—where Hengrui Pharma manufacturers camrelizumab—and India, had raised concerns and led to delayed regulatory approvals for other pharmaceutical firms. Yet Elevar’s regulatory affairs staff thought it was time to discuss precise wording of the C+R drug labels and learn what Prescription Drug User Fee Act (PDUFA) fees the FDA would assess. Elevar Therapeutics agreed to pay Hengrui $600 million in milestone and royalty payments to market C+R outside of China and Korea [16].
On July 9, 2024, Elevar announced near term plans to resubmit its new drug application (NDA) for C+R to treat uHCC following a Type A meeting with the FDA held on July 2, 2024. Type A meetings are requested from the FDA when a stalled drug development program seeks a critical path to proceed or address an important safety issue. In its press release, Elevar stated, “Prior to the Type A meeting, the FDA had accepted Hengrui Pharma’s written responses to GMP deficiencies. During the meeting, the FDA confirmed resubmission can occur without delay. The FDA also confirmed BIMO inspections due to FDA travel restrictions may occur after resubmission” [17] A reasonable interpretation of the Elevar press release would be the FDA is now satisfied or maybe the delay was due to a misunderstanding, and Elevar will resubmit its NDA the following week. Fierce Pharma reported that Elevar first intended to refile its NDA by October 2024 and then revised the timing for the resubmission by the end of the year [18]. “Investors, who were clearly disappointed in that cautious estimate, dragged down the stock price of Elevar’s Korean parent company, HLB” [18]. A simple class 1 resubmission normally takes the FDA two months to review, but for a more elaborate class 2 resubmission, e.g., with new clinical data, the FDA requires six months or longer to review. Elevar stated that it intends to add the data from the final analysis in which the median OS time for the C-R treated group was revised from 22.1 to 23.8 months compared to 15.2 months for the soranib-treated group [13]. The FDA may consider the new clinical data as triggering a class 2 resubmission with a six-month review cycle [18], which could delay an approval decision until July 2025.
The CARES-310 clinical trials produced a new longest median OS of 23.8 months for patients with uHCC treated with C-R. However, the CARES-310 clinical trial may have been overweighted with an Asian population with preexposure to antiviral therapy and better preservation of liver function at time of HCC diagnosis. Conversely, the CARES-310 trial was underweighted with Western patients who have NAFLD, diabetes, obesity, and other co-morbidities and confounding factors. Oncologists already have 1L patient treatment experience for uHCC with Roche’s FDA-approved anti-PD-L1/VEGF combination Tecentriq and Avastin and with AstraZeneca’s CTLA-4 checkpoint inhibitor Imjudo combined with its PD-L1 antagonist Imfinzi for treatment of uHCC. It would be useful to compare the C-R proposed therapy with these drug combinations and determine if C-R maintains an 8.6-month median OS advantage over the new comparator(s). When rivoceranib plus hospice care was compared to hospice care alone in a clinical trial of patients with advanced gastric cancer or gastroesophageal adenocarcinoma, the results data were mixed. For some measures of functional benefits and harms, the patients with hospice care alone had higher percentages for certain functional benefits and lower percentages for some harms and adverse events.
The rivoceranib-treated group failed to consistently outperform hospice care alone using these measures. Based on those benefits and harms, hospice care alone was not inferior to rivoceranib + hospice care, which calls into question the underlying clinical value, if any, of rivoceranib to the American patient population with advanced gastric cancer. The fact that rivoceranib, known as apatinib, is approved in China for treatment of advanced gastric raises a new questions about fundamental differences in patient populations between China and the USA or Europe for medical conditions such as uHCC and advanced gastric cancer.
Christopher Coleman, Pharm.D., provided useful comments.
None.


