Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Isupova AA*
Received: July 17, 2024; Published: July 25, 2024
*Corresponding author: Isupova AA, Department of Dermatovenereology and Phthisiology, Kyrgyz-Russian Slavic University, 44 Kyivstreet, Bishkek, Kyrgyz Republic, 720000, krsu.edu.kg, Kyrgyzstan
DOI: 10.26717/BJSTR.2024.57.009062
The epidermal barrier is the most important component of human skin, which provides protection from
environmental stressors, including radiation. Due to the high rate of cell renewal and the abundance of dividing
cells, the epidermis is particularly vulnerable to toxic and radiation-induced damage. This article discusses the
effects of long-term (more than 50 years) endogenous intake of radionuclides on the function of the epidermal
barrier in people living in the vicinity of uranium tailings dumps. Comprehensive studies of the content of
radionuclides in water, soil, meat of cattle and small cattle, conducted within the framework of the ISTC, prove
the intake of radionuclides into the human body through the food chain. For the period from 2003 to 2024, skin
studies were conducted in people living in various regions of Kyrgyzstan. A total of 4184 people were examined.
The main group (603 people) is people living in the vicinity of uranium tailings dumps. This message reflects
only part of the overall work related to the health of people living near uranium reservoirs.
Conclusion: Long-term exposure to low doses of radiation leads to changes in the human integumentary
system. Understanding these changes can help develop strategies to prevent or mitigate the harmful effects of
radionuclides on human health in the event of a radiation hazard.
Keywords: Radionuclides; Ecology; Mountains; Etiology and Pathogenesis; Skin; Dermatitis; Epidermal Barrier; Human Integumentary System; Uranium Tailings; Technogenic Pollution
Abbreviations: ATR: Attributable Risk; RR: Risk Ratio; NNT: No-Tolerance Index; SOD: Superoxide Dismutase; ROS: Reactive Oxygen Species; NMF: Natural Moisturizing Factor; ATM: Ataxia-Telangiectasia Kinase; SD: Standard Deviation; SE: Standard Error; CI: Confidence Interval
On the Role of Control and Regulation of Anthropogenic Pollution Factors
Exposure to radiation can occur from various sources, including medical procedures, nuclear tests and accidents, and occupational hazards. In the case of anthropogenic pollution, one of the most common sources of exposure is the ingestion of radionuclides through contaminated water, food, and air. According to the reports of the International Atomic Energy Agency, modern technologies for the disposal of uranium processing tailings have reached a certain maturity [1] However, some uncertainty remains regarding the long-term efficiency of these tailings’ facilities. The tailings facilities themselves pose significant challenges to ensure their long-term stability: controlled consolidation and minimization of subsequent release of pollutants. Successful stabilization of legacy sites faces a number of challenges, such as soil erosion caused by wind and water, before any cover can provide long-term protection. Modern conditions require the development and extraction of energy resources. Therefore, it is extremely important to control and regulate emissions of man-made pollutants to minimize human impact and maintain the ecological balance of the Earth.
Radionuclides and their Impact on the Epidermal Barrier
Radionuclides emit ionizing radiation, which damages biological tissues. The epidermis is particularly susceptible to radiation damage due to its high renewal rate and the presence of dividing cells. Studies have shown that low-dose radiation exposure can lead to changes in epidermal barrier function, including increased permeability, changes in lipid composition, and impaired cell proliferation [2,3]. These changes can lead to dry, itchy, and inflamed skin. One of the main mechanisms by which radionuclides affect the epidermal barrier is oxidative stress. Ionizing radiation generates reactive oxygen species (ROS), which can damage cellular components, including DNA, proteins, and lipids [4]. ROS can also induce apoptosis, resulting in a decrease in the number of keratinocytes, the predominant cell type in the epidermis. In addition, ROS can disrupt the lipid bilayer of the SC, leading to increased permeability and water loss [5]. Another mechanism by which radionuclides affect the epidermal barrier is through changes in the expression of genes involved in barrier function. For example, studies have shown that low-dose radiation exposure can reduce the expression of filaggrin, a protein that plays a critical role in maintaining the integrity of the stratum corneum [6]. Filaggrin deficiency results in a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. Filaggrin breakdown products are known to promote the formation of natural moisturizing factor (NMF), which helps retain water in the stratum corneum [7].
Decreased filaggrin expression may result in decreased NMF production, leading to dry, itchy skin and inflammation skin [8]. In irradiated skin, hyperactivation of proinflammatory signaling pathways is observed, leading to keratinocyte hyperproliferation and thus to epidermal hyperplasia [9]. The effect of radiation-induced skin damage on hyaluronic acid degradation and its underlying mechanisms [10].
Natural Defense Mechanisms
In response to DNA damage, cells activate a complex network of signaling pathways, including the ataxia-telangiectasia kinase (ATM) pathway, which regulates cell cycle arrest, DNA repair, and apoptosis. In the epidermis, activation of the ATM pathway leads to phosphorylation of p53, a tumor suppressor protein that promotes cell cycle arrest and apoptosis in response to DNA damage. Activation of p53 also leads to upregulation of p21, a cyclin-dependent kinase inhibitor that prevents cell cycle progression and allows time for DNA repair [11,12]. Most tissues and organs possess a set of defense mechanisms that interact with toxic substances to eliminate the harmful effects of ROS [13]. They include both enzymatic and nonenzymatic molecules that function as potent antioxidants or oxidant-scavenging systems [13]. ROS are scavenged from the cell by enzymatic systems, including superoxide dismutase (SOD), catalase, aldehyde dehydrogenase, and glutathione peroxidase, or by nonenzymatic systems, including antioxidant vitamins such as alpha-tocopherol and ascorbic acid. Glutathione peroxidase reduces hydrogen peroxide by transferring the energy of reactive peroxides to a small sulfur-containing protein, glutathione. Other known protective substances include peroxiredoxin, which together with SOD provides a potent system against damaging ROS metabolites [14].
Geographical Conditions of Residence and General Characteristics of Technogenic Pollution of the Mountain Ecosystem of Kyrgyzstan
The country’s territory is 93% mountainous with a height of 1000–7400 m. The average height above sea level is 2750 m. More than 40 percent of the country’s territory is located at an altitude of over 3000 m, and three quarters of it is covered with permanent snow or glaciers, with 600 glaciers covering an area of 6578 km2 [15]. A total of about 400,000 m3 of industrial waste and equipment is buried on the territory of the republic. After the cessation of activities of mining and processing enterprises for the extraction and processing of uranium, 35 of the 49 tailings dumps that formed were abandoned. Of the 80 dumps of substandard uranium ores, 25 were abandoned [16]. Currently, there is an economic need to resume the mining of uranium and some minerals. In this regard, the data of this study become particularly relevant.
A total of 4,184 people living in various regions of Kyrgyzstan were examined from 2003 to 2024. The risk group consisted of 603 people living near uranium tailings dumps in the mountains; the comparison group consisted of people living in areas free of radionuclides. The skin was examined using standard methods. Initially, the condition of outwardly unchanged areas of the skin was assessed, and then the lesions were described. Skin color, the presence of de- and hyperpigmentation were determined, the place where these changes were most pronounced was indicated; elasticity, turgor and hydration (normal, dry, moist skin). In the presence of rashes, their nature (inflammatory or non-inflammatory), symmetry, prevalence of the process, localization of rashes, damage to open or closed areas of the skin was assessed. To analyze the clinical and epidemiological studies, a specially developed card was filled in based on the information obtained during the study of outpatient cards, clinical and instrumental methods of skin examination of the selected groups. During the interview, a questionnaire consisting of 54 questions was filled in. Epiluminescence microscopy of skin neoplasms was performed using a Heine Delta 20 dermatoscope (K 256.27.376, Heine Optotechnik, Germany), emission medium (Dermatoscopy Oil, K-00.34.005, Heine Optotechnik, Germany) and a recording device - a Nikon 5300 digital camera (Japan); a photo adapter designed for contact with the HEINE DELTA 20/Nikon Coolpix writing device (K-00.34.235, Heine Optotechnik, Germany). Dermoscopic assessment of melanocytic pigmented skin lesions was performed using “pattern analysis”. Panoramic skin scans were performed under standard conditions.
Data Collection and Use of Variables
All subjects signed informed consent to participate in the study. All ethicalstandards and principles of confidentiality were observed during the study in accordance with the principles of the Helsinki Declaration of the World Medical Association, as amended in 2000, “Ethical Principles for Medical Research Involving Human Subjects”.
Statistical Analysis
Statistical analysis was performed using PASW Statistics 21.0 (SPSS Inc., IBM, Chicago, USA). Descriptive statistics are presented as mean, Standard Deviation (SD), and Standard Error (SE). Statistical significance of variables was determined using the Pearson chi-square test. All tests are two-sided, and p < 0.05 was considered statistically significant. The contingency coefficient phi Cramer̕ s V was used to assess the relationship of nominal data, where the value varies from 0 to 1, with “0” indicating no relationship between the row and column variables, and a value close to 1 indicating a high degree of relationship between these variables. To assess the effect of risk factor exposure, the Attributable Risk (AtR), Risk Ratio (RR) and No-Tolerance Index (NNT) were calculated for each variable. The 95% confidence interval (95% CI) was calculated for all variables. Statistical significance was determined using the Cochran and Mantel- Haenszel tests, where p < 0.05 was considered statistically significant. After Bonferroni correction (when >2 groups are compared), the significance level was p < 0.01.
355 indigenous people of Kyrgyzstan were examined. Of these, 62 (17.5%) people were excluded from the study according to the exclusion criteria (Table 1). Table 1 shows the exclusion factors that could potentially affect the study results. Table 2 presents the descriptive statistics of the study groups. The sample consisted of 293 people: the risk group - 75 people (25.6%), the comparison group - 218 (74.4%) people. The risk group included 30 (40%) men and 45 (60%) women, with an average age of 38.7 ± 14.7 (95% CI 35.3-42.1) years, age range from 17 to 75 years. The comparison group included 99 (45.4%) men and 119 (54.6%) women, with an average age of 38.0 ± 15.4 (95% CI 36.0-41.1) years, age range from 17 to 77 years. The data obtained as a result of the study were stratified by the presence or absence of stigmas of epidermal barrier disruption (Table 3). Within the risk group and the comparison group, the number of subjects with signs of seborrheic keratomas, senile angiomas, acrochordons, clinical signs of xerosis, and skin inflammation was calculated. As a result of the calculations, a four-field contingency table was obtained (Table 3), according to which the frequency of occurrence of the main objective stigmas of epidermal barrier disruption was calculated. To determine the relationship between the risk factor and pathological changes in the human integumentary system, we calculated the attributable or additional risk indicators (Table 3). The attributable risk shows how much the risk factor increases the probability of the pathological process, in addition to the probability that exists for individuals who have not been exposed to the factor under study.
Table 3: Frequency rates of occurrence of stigmas of human integumentary system disorders in the risk group and in the comparison group.
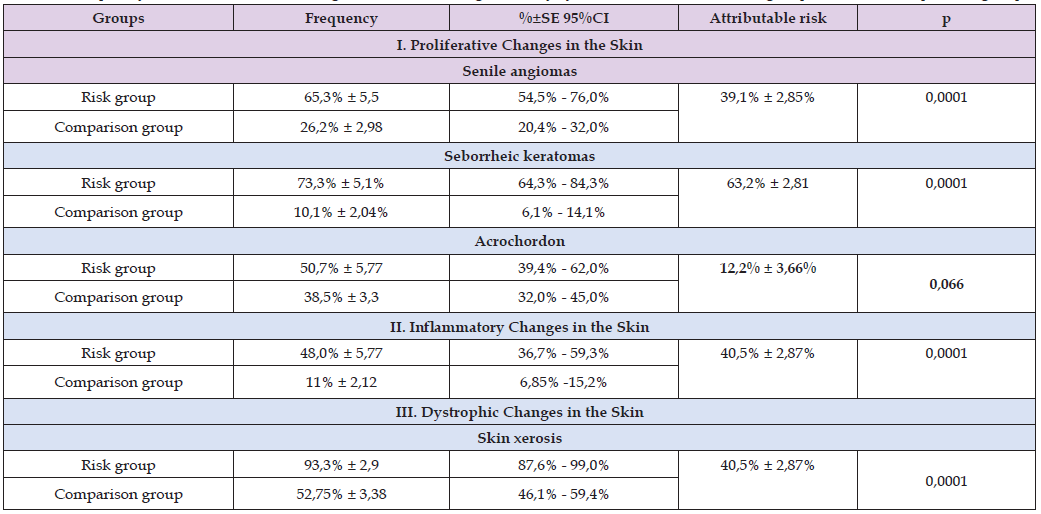
Thus, the risk of developing inflammatory skin changes is 37% ± 2.82% (95% CI 31.5% - 42.5%) higher than in individuals not exposed to the risk factor (Table 3). The probability of developing increased dry skin (xerosis) is 40.5% ± 2.87% (95% CI 34.4% - 45.6%) higher than in the control group (Table 3). Under the influence of the risk factor, the probability of developing seborrheic keratomas and senile angiomas exceeds 63.2% ± 2.81 (95% CI 57.7% - 68.7%) and 39.1% ± 2.85% (95% CI 33.5% - 44.7%), respectively, compared to the control (Table 3). These calculations confirm the high value of the identified diagnostic criteria for assessing the impact of technogenic pollution. Calculation of the contingency coefficient phi Cramer̕’s V (Table 4) shows the strength of the relationship between the studied variables and the risk factor. From Table 3 it is clear that for acrochordon the relationship with the studied risk factor is weak (0.107), and according to the Pearson coefficient this relationship is statistically insignificant (p=0.066). This indicates the absence of a relationship between long-term endogenous exposure to radionuclides on the human body and the formation of acrochordon. The strongest correlation with the risk factor was found in the calculations of seborrheic keratosis indices – 0.627. The calculations showed that inflammatory skin changes (0.400), xerosis (406) and senile angiomas (0.356) have a moderate correlation with the risk factor (included in the range from 0.3 to 0.6). For all variables except Ach, Pearson’s chi-square coefficient calculations (Table 3) indicate that the probability of the “null hypothesis” is less than 0.0001.
Table 4: Frequency of occurrence of the main objective stigmas of disruption of the epidermal barrier and microcirculatory bed in individuals living near uranium tailings dumps and in the comparison group.
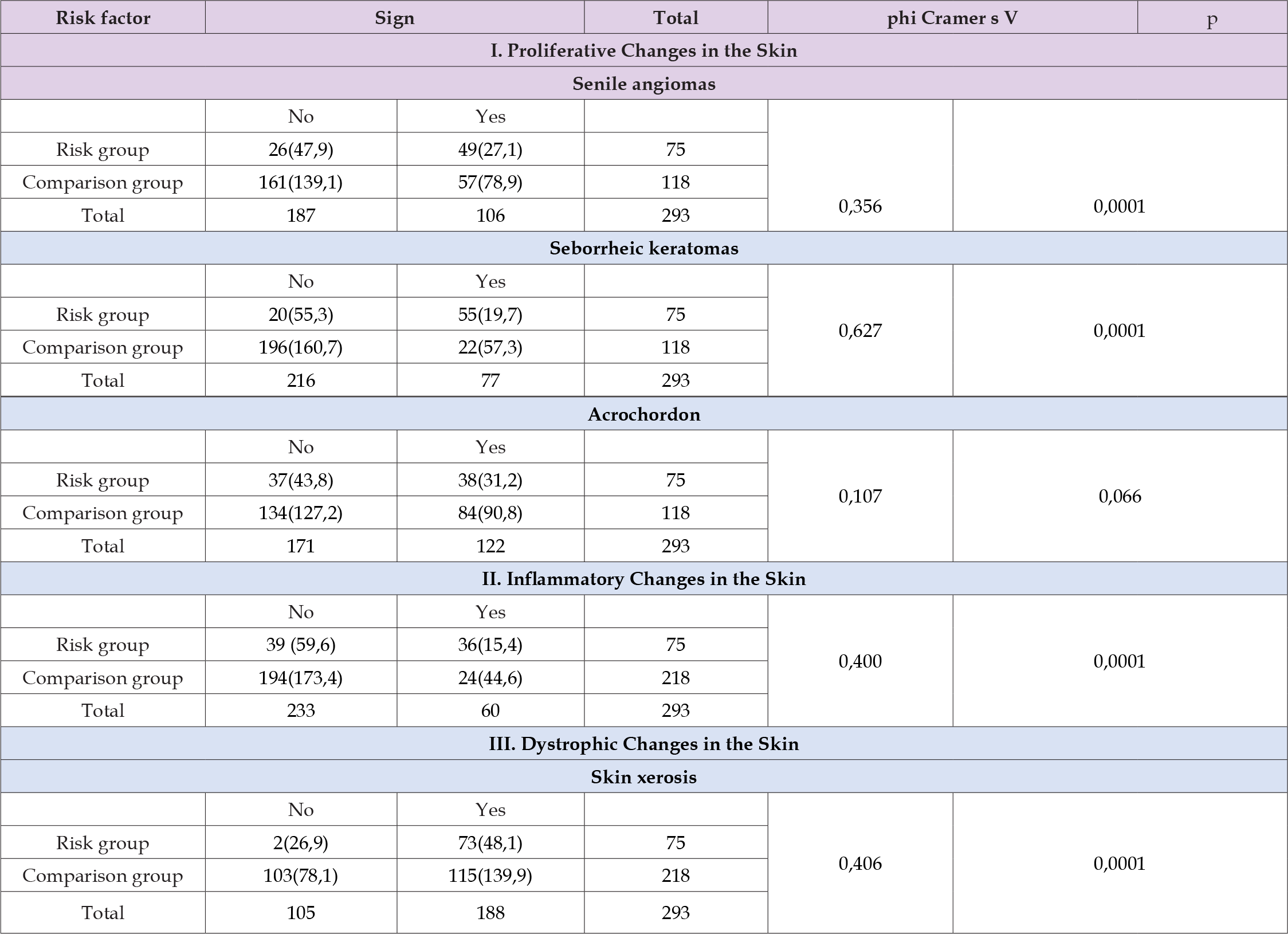
Note: In brackets is the expected frequency in the case of assumed equality of living conditions.
The risk ratio (RR) (Table 5) shows how many times the frequency of occurrence of seborrheic keratomas, senile angiomas, dry skin and skin inflammation among individuals exposed to the risk factor differs from individuals in the control group. Thus, the probability of occurrence of skin xerosis is 4.4 times, skin inflammation is 1.9 times, seborrheic keratomas are 7 times, and senile angiomas are 2.5 times higher than the indicators of these variables in the control group (Table 5). The RR indicators for acrochordon are statistically insignificant, which confirms the lack of diagnostic value of this criterion. The RR calculation shows the gradation of sensitivity of the selected diagnostic criteria for assessing the condition of the skin during long-term endogenous exposure to radionuclides.
Table 5: Indicators of the probability of developing an adverse outcome in people living near uranium tailings dumps.
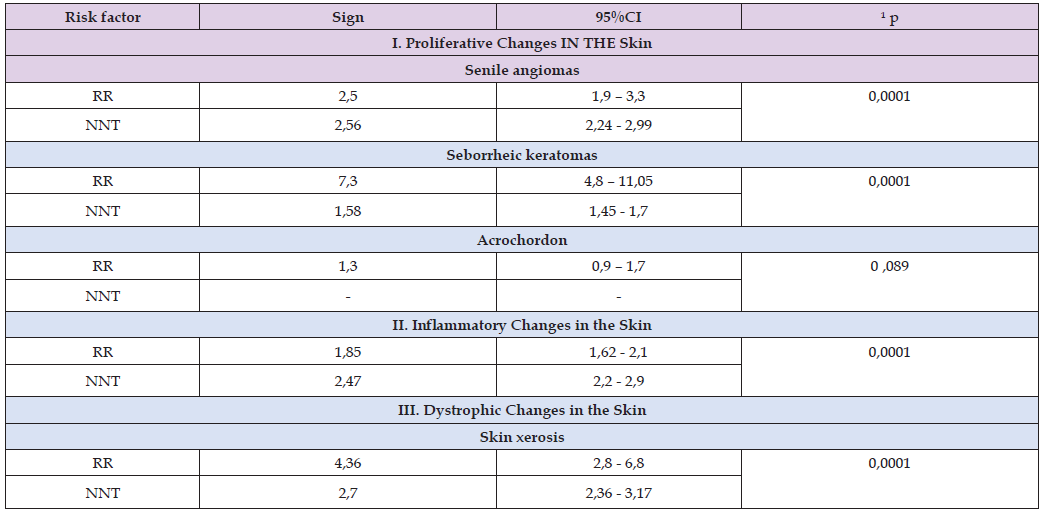
Note: ¹Cochran and Mentel-Haenzel test
Long-term, multi-year exposure of the internal environment and integumentary system of the body to radionuclides has a number of clinical consequences. Radiation-induced and toxic damage to the epidermis can have significant clinical consequences, including dry skin, peeling, itching, increased risk of infection and uncontrolled proliferative processes, which are the main risk factors for the development of skin neoplasms. Prevention and early detection of radiation damage to the epidermis are crucial to minimize morbidity and even mortality. Personal prevention strategies include wearing protective clothing in the workplace, maintaining good hygiene and a healthy lifestyle, regular self-examinations and annual skin examinations by a dermatologist. State strategies should be aimed at minimizing the spread of man-made waste; implementing safety measures in the workplace; monitoring the content of radionuclides in soil, water, air and food; creation of treatment facilities for drinking water, as well as water for irrigation of vegetable gardens and orchards in the immediate vicinity of uranium tailings dumps. Provision of timely and adequate medical care to the population.
Professional orientation of health workers in mining areas, availability of modern means of radiation protection and prevention are important. Access to the use of local means that can improve the function of the epidermal barrier. For example, moisturizers, emollients and occlusive agents that reduce transepidermal water loss. These steps are simple and very important for maintaining healthy skin. Thus, knowledge of the skin condition of people living in areas of technogenic contamination and understanding the mechanisms of these changes can help in developing strategies to prevent or mitigate the harmful effects of radionuclides on human health in the event of a radiation hazard.
The author confirms that there is no conflict of interest.


