Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Tee Yi En, Nabila Perveen and Naeem Hasan Khan*
Received: July 15, 2024; Published: July 24, 2024
*Corresponding author: Naeem Hasan Khan, Faculty of Pharmacy, AIMST University, 08100 Bedong, Kedah D.A., Malaysia
DOI: 10.26717/BJSTR.2024.57.009055
Allium sativum, commonly known as garlic, is a medicinal plant with a rich history of traditional use. It belongs
to the Liliaceae family and is renowned for its health benefits, attributed to its phytochemical composition,
particularly sulfur-containing compounds like allicin. This study aims to explore the phytochemical profile of
garlic, its antibacterial activity against selected bacterial strains and its antioxidant properties.
Objectives: To conduct phytochemical screening of Allium sativum and evaluate the antibacterial activity of
garlic extract against Bacillus subtilis, Staphylococcus epidermidis and Escherichia coli, as well as to assess its
antioxidant properties using the DPPH assay.
Method:
i. Maceration extract of Allium sativum was carried out by using 95% ethanol as the solvent to obtain ethanol
extract.
ii. Phytochemical screening was performed using standard qualitative tests to detect the presence of alkaloids,
flavonoids, terpenoids, glycosides, tannins, saponins, anthraquinones and carbohydrates.
iii. Antibacterial activity was tested using the well diffusion method on Mueller-Hinton Agar plates with
ciprofloxacin as a positive control.
iv. Antioxidant activity was determined using the DPPH scavenging method with ascorbic acid as a standard.
Result: Phytochemical screening revealed the presence of alkaloids, terpenoids and saponins in the ethanolic
garlic extract, while other compounds were absent. The antibacterial tests showed no inhibition zones against
the tested bacterial strains at the concentrations used (50 mg/mL and 100 mg/mL), indicating no antibacterial
activity under the experimental conditions. The antioxidant assay demonstrated that the ethanolic extract of
garlic extract has a strong free radical scavenging ability, with an IC50 value of 1.41 mg/mL, indicating potent
antioxidant properties.
Conclusion: The study concludes that Allium sativum contains phytochemicals with potential health benefits,
particularly as an antioxidant agent. The absence of antibacterial activity in this study suggests that the
concentrations tested, or the extraction method used may not have been sufficient to elicit a response against
the selected bacterial strains. Further research is recommended to optimize extraction methods and explore
higher concentrations to fully understand the antibacterial potential of garlic.
Keywords: Allium Sativum; Garlic; Phytochemicals; Antibacterial; Antioxidant
Allium sativum (A.S.) is also commonly known as garlic, is a type of plant belonging to Liliaceae family and is among the oldest of all cultivated plants (Alam, et al. [1]). A.S. is the most widely researched medicinal plant and has been used for about 4000 years as a traditional remedy to treat or control varies conditions like the common cold, high blood pressure, preventing blood clot etc.
Botanical Description
A.S. normally grows around 60 cm height. The leaves are flat, long and narrow with an acute apex which is attached to an underground stem. The underground bulb of garlic is made up of cloves. These cloves are prophylls, enclosed by dry membranous skin and relate to basal plate as shown in Figures 1- 3. A.S. can be cultivated via a sexual method from the cloves, where pollinator is not required. It will grow well during a cool and moist season in well-drained soil. As garlic is matured, relatively dry period will be needed. Some conditions like warm temperature, low relative humidity and airflow conditions allowed the process of drying neck tissues of cloves and outer leaf sheaths that will be done after harvesting more efficiently. Moreover, this process can prolong the postharvest life and quality of garlic.
Chemistry of Allium Sativum (A.S.)
A.S. consists of various kinds of sulfur-containing compounds with different significances and these compounds give garlic its characteristic odor and favor as well as some favorable effects. Sulfur-containing compounds present in garlic include alliin, allicin, ajoene, 2- Vinyl-4H-1,3-dithiin, Diallyl sulfide, Diallyl disulfide, Diallyl trisulfide and Allyl methyl sulfide as shown in Table 1. and further in Figures 4 & 5 (Mardomi, et al. [2]). Glutamyl cysteines are the main sulfur components in intact garlic clove (Powolny, et al. [3]). Alliin can be produced via hydrolysis of glutamyl cysteine. Alliin can accumulate naturally during bulbs storage under cool temperature. Enzyme alliinase will react with alliin to produce allicin. This occurs during garlic crushing via chopping or chewing. Allicin is the major compound that give garlic its characteristic odor. Approximately 70- 80% of thiosulfinates in Allium sativum are allicin which is a diallyl thiosulfinate. Moreover, allicin is an unstable compound which can break down and yield other sulfur compound including allicin, a diallyl thiosulfinate that accounts for 70-80% of the thiosulfinates present in Allium sativum (Amagase, et al. [4]). Allicin is also highly unstable and quickly decomposes to yield sulfur compounds when oxidized such as DAS, DADS, DATS, ajoene, and hydrogen sulfide (Alam, et al. [1]). Glutamyl cysteine also decomposes into S-allyl-cysteine (SAC) by another pathway. SAC is an important garlic component which gives the health benefits of garlic.
Significance of Allium sativum
A.S. is one of the most common plants that has been widely used in medicine areas since ancient times. Bioactive components of garlic, allicin and its derivatives have been shown to be used in many types of diseases with favorable effect. Firstly, some studies have demonstrated that Allium sativum is able to reduce blood sugar levels in diabetic animals. S-allyl cysteine sulfoxide, one of the active ingredients from garlic, will produce hypoglycemic effect by stimulating insulin secretion (Srinivasan, et al. [5]). Other than hypoglycemic effect, garlic also has cholesterol lowering effect, which is useful for hypercholesterolemia conditions. According to Santhosha et al. (2013), garlic oil, allicin and ajoene can lead to reduction in cholesterol biosynthesis. So, garlic can be an adjunct therapy for people with high cholesterol levels as it can act as cholesterol lowering agent. Furthermore, Allium sativum also useful in curing several body health conditions, like asthma, fever, malaria, backache, rhinitis, arthritis as well as some skin disease such as leprosy, itches, leukoderma and more (Pendbhaje, et al. [6]). Garlic is also popular for its aromatic characteristics which are provided by organosulfur components which include allicin and DADS. So, it has been widely used in numerous cuisines worldwide as favoring agent. The Figure 6 shows the pharmacological actions and mechanisms.
Phytochemical Screening
(Figures 7a & 7b) (Lanzotti, et al. [7]) Phytochemicals are defined as bioactive compounds which are naturally derived from plants for their defense (Kumar, et al. [8]). The conversion of alliin to allicin has been shown in Figures 7a & 7b. These compounds can be obtained from wide range of sources such as fruits, herbs, vegetables, and other plant-based products. To date, there have been more than a thousand phytochemicals that have been found from various studies. Some examples of important phytochemicals compounds are polysaccharides, polyphenols, flavonoids, carotenoids and more. Most phytochemicals compounds have properties to exhibit antioxidant, antibacterial, anticancer, and antispasmodic activities. Figures 7a & 7b shows the biosynthesis of alliin to allicin and main saponin from bulbs. The presence of phytochemicals in A.S. can be identified via process of phytochemical screening, which involve process of identifying and analyzing the bioactive components present in A. Sativum. A series of qualitative and quantitative tests can be carried out for detection and confirmation of presence of phytochemicals like alkaloid, flavonoid, glycosides and terpenoids. A series of screening test done can help in obtaining deeper understanding on bioactive components inside garlic which will be very helpful in many types of applications especially in pharmaceutical field as therapeutic agents.
Antioxidants Activity
Antioxidants are defined as substances that can give protection for cell bodies against free radicals. Antioxidants act by neutralizing free radicals which may are unstable chemicals that can harm cells and tissues through oxidative stress, that can result in several health problems. The most significant components of defense systems of the body are antioxidant enzymes. Antioxidant enzymes can control mechanisms that regulate oxidative stress pathways. The phytochemicals that have antioxidants properties also aid in regulating the oxidative stress pathways. A.S. exhibits antioxidant properties due to the presence of unstable and irritating organosulfur compounds of garlic (Capasso, et al. [9]).
Antibacterial Activity
Antibacterial agents refer to substances from plant extract or any chemical compounds that can fight against pathogenic bacteria. Hence, reducing the harm brought by bacteria to the biological systems via eliminating or minimizing the metabolic activities of bacteria. A.S. also acts as a potent antibacterial agent due to its rich composition of bioactive compounds, especially those sulfur-containing compounds and other phytochemicals. It is found that allicin, E-ajoene and many other aliphatic sulfides are primary antibacterial organosulfur components in A.S. (Bhatwalkar, et al. [10]). Among these aliphatic sulfides, allicin is the major component which has been used in research to understand its antibacterial activity. It was found that allicin possesses antibacterial activity against a wide range of bacteria, including both gram-positive and gram-negative strains (Ankri, et al [11]). Figure 8 shows antibacterial effects of garlic and gentamycin as control on different bacteria strains (Magryś, et al. [12]).
The main objective of this research is to conduct a comprehensive screening of phytochemicals found in Allium sativum (garlic). In addition, the study also intends to assess the antibacterial activity of garlic extract against different bacterial strains as well as to evaluate the antioxidant properties of garlic extract.
Selection of Plant Materials and Extract Preparation
Fresh Allium sativum (A.S.) (garlic) was collected from the local fresh vegetable market. Firstly, the cloves of garlic were cut, followed by crushing the cloves by using mortar and pestle. Then, the crushed garlic was soaked in ethanol for at least 24 hours. Ethanol is used because it is less toxic and able to preserve bioactive chemicals that are present inside garlic. The mixture was placed on the shaking incubator for maceration process. After 5 days, the mixture of fresh garlic with ethanol was filtered and squeezed out for the solvent. Then, the extract was allowed to evaporate by using rotary evaporator, followed by transferring to evaporating dish and kept at water bath with temperature of 60°C for solvent evaporation.
Preparation of Allium Sativum Ethanol Extract
A stock solution of 3mg/ml was prepared by dissolving 300mg of extract in 100ml of ethanol.
Antibacterial Assay
Three petri dishes were used for each bacterial strain, so a total of nine petri dishes were used in this antibacterial study. Each petri dish was divided into four regions, which are positive control, negative control, and with two different concentrations of garlic extract. 50mg/ml and 100mg/ml of garlic extract is prepared by using volumetric flask. The well diffusion method was used. All the selected bacterial stains were allowed to grow on Mueller-Hinton Agar (MHA) at 37 °C. To achieve effective diffusion, bacterial suspension was applied to Mueller-Hinton agar plates using an L-shaped spreader. After allowing the plates to dry for 15 minutes, an approximately 6-mm diameter well was created using a cork borer in each region. Hence on each plate, there were total of four wells, one well filled with positive control which is ciprofloxacin, distilled water was used as negative control in another well, then the rest two wells were filled with 50mg/ml and 100mg/ml of garlic extract, respectively. Following the incubation period of 24 hours at 37 °C, the diameter of the inhibition zone was recorded, and plates were photographed.
Preparation of Different Concentrations of Gallic Acid Standard
Gallic acid was used as the standard for this assay. Six different concentrations of standard solution were prepared in 3.0mg/ml, 2.4mg/ml, 1.8mg/ml, 1.2mg/ml, 0.6mg/ml and 0.3mg/ml. Serial dilution methods were used to prepare the various concentrations. A stock solution of 3mg/ml was prepared by dissolving 100mg of garlic acid in 100ml of ethanol. Then, from the stock solution, 10, 8, 6, 4, 2 and 1ml were pipette out into the volumetric flask and made up to 10ml by adding ethanol individually to produce different concentrations of 3.0mg/ml, 2.4mg/ml, 1.8mg/ml, 1.2mg/ml, 0.6mg/ml and 0.3mg/ml. More elaboration is shown in Table 2.
Total Phenolic Content Test
A 2.5% sodium carbonate solution was prepared by dissolving 2.5g of sodium carbonate in 100mL of distilled water. 1mL of ethanol extract was added into the test tube by using micropipette. Then, 1mL of Folin-Ciocalteu reagent and 2mL of 2.5% sodium carbonate solution. For standard gallic acid, 1mL each from various concentrations of solution was added with 1mL of Folin-Ciocalteu reagent and 2mL of 2.5% sodium carbonate solution in a test tube, respectively. Control solution was prepared by replacing the extract with 1mL of ethanol and addition of 1mL of Folin-Ciocalteu reagent and 2mL of 2.5% sodium carbonate solution. 3mL of ethanol was added into another test tube which acted as blank solution. All test tubes that contained various concentrations of standard garlic acid, extract, control and blank were allowed to stand for 2 hours. Next, 2mL of each test tube’s content was subjected to UV visible analysis. The absorbance was measured at 760nm in UV- Visible spectrometer. The absorbance values of each different concentration of standard garlic acid, control and blank were recorded. The total phenolic content was expressed in mg of garlic acid equivalents per g dry extract (mg GAE/g). All points were plotted to line graph where x-axis was the concentration of garlic acid and y-axis was absorbance. The formula below was used to calculate the total concentration of phenolic content (Genwali, et al. [13]).

Where, C= total phenolic content mg GAE/g dry extract,
c= concentration of garlic acid obtained from calibration curve in
mg/ml,
V= volume of extract in mL,
m= mass of extract in gram.
Evaluation of Antioxidant Activity
The DPPH scavenging method was used for evaluation of antioxidant activity.
Preparation of Different Concentrations of A. Sativum ethanol extract: Six different concentrations of extract solution were prepared in 3.0mg/ml, 2.4mg/ml, 1.8mg/ml, 1.2mg/ml, 0.6mg/ml and 0.3mg/ml. 300mg of garlic extract was dissolved in 100mL of ethanol, the mixture acted as stock solution. From the prepared extract solution, 10mL, 8mL, 6mL, 4mL, 2mL and 1mL were pipetted out into the 10mL volumetric flask and the volume was made up to the calibration line of volumetric flask by adding ethanol, respectively (Table 3).
Preparation of Different Concentration of Ascorbic Acid Standard: Ascorbic acid was acted as standard for this study. The concentration of 3.0mg/ml, 2.4mg/ml, 1.8mg/ml, 1.2mg/ml, 0.6mg/ml and 0.3mg/ml of the standard solution was prepared. The concentration was prepared by serial dilution methods. Stock solution was prepared by dissolving 300mg of ascorbic acid into 100mL of ethanol. From the prepared stalk solution, 10mL, 8mL, 6mL, 4mL, 2mL and 1mL were pipetted out into the 10mL volumetric flask and the volume was made up to the calibration line of volumetric flask by adding ethanol, respectively, as shown above in Table 3.
DPPH Antioxidant Assay
2,2-diphenyl-1-picrylhydrazyl (DPPH) was prepared by dissolving 11.83mg in 100mL of ethanol. 2.5mL of different concentration of prepared extract was added into different test tube respectively. Then, aluminum foil was used to cover all the test tubes due to DPPH is a light photosensitivity reagent. Then, 1mL of DPPH solution was added into each test tube. Then, the control solution was prepared with 2.5mL of ethanol and 1mL of DPPH solution. 3mL of ethanol was used as blank solution. All the test tubes filled with different concentrations of ethanol extract, standard ascorbic acid, control and blank were allowed to stand inside dark cupboard for 30 minutes. After 30 minutes, all prepared samples were inserted into a spectrophotometer, and measured the absorbance between 400 and 750 nm (Rahman, et al. [14]). The absorbance values of each concentration of the extract and ascorbic acid standard, as well as control were recorded as given in Table 4.
The Percentage of DPPH Scavenging Effect was Calculated by Following Equation:
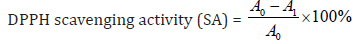
Where A0: absorbance of control;
A1: absorbance of sample.
Then, the graph for DPPH assay for both A.S. ethanol extract and ascorbic acid standard were plotted by percentage of scavenging activity versus concentration. The equation that was generated by both the graphs allowed the value of IC50 which indicated the sample concentration that reduced the initial DPPH absorbance of 50%.
Phytochemical Screening of Allium Sativum Ethanol Extract
Table 5 is laid down with the results of phytochemicals present in ethanol extract of Allium sativum.
Antibacterial Activity of Allium Sativum Ethanol Extract
Antibacterial Activity Against Bacillus Subtilis: Results of Figure 9: A. sativum ethanol extract showed negative result towards Bacillus subtilis as no zone of inhibition observed from both concentration of the extract in all three petri plates. Only positive control, which is ciprofloxacin showed zone of inhibition.
Antibacterial Activity Against Staphylococcus Epidermidis: Results of Figure 10: A. sativum ethanol extract showed negative result towards Staphylococcus epidermidis as no zone of inhibition observed from both concentration of the extract in all three petri plates. Only positive control, which is ciprofloxacin showed zone of inhibition.
Table 6: Table of antibacterial activity of Allium sativum ethanol extract in 50mg/ml and 100mg/ml concentration with positive and negative control.

Detection of Total Phenolic Content (TPC)
Gallic Acid (Standard Solution): Absorbance concentration of gallic acid standard and absorbance values are shown in Table 7 while the absorbance verses concentration of gallic acid standard is shown in Figure 12.
A. S. Ethanol Extract: The absorbance value of extract with 3mg/ ml obtained is 0.235.
Evaluation of Antioxidant Activity of Allium Sativum Ethanol Extract
Concentration of ascorbic acid standard, absorbance value of control and ascorbic acid standard with scavenging activity (SA) is shown in Table 8 and percentage scavenging against concentration of ascorbic acid is given in Figure 13, respectively. Table 9 shows the concentration of A. sativum ethanol extract, absorbance value of control and extract with scavenging activity (SA) and DPPH percentage scavenging against the concentration of ascorbic acid standard in Figure 14, respectively.
Table 8: Concentration of Ascorbic Acid Standard, Absorbance Value of Control and Ascorbic Acid Standard with Scavenging Activity (SA).
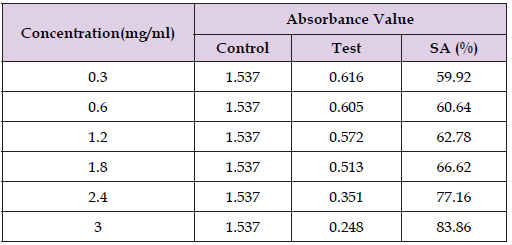
Table 9: Shows the concentration of A. sativum ethanol extract, absorbance value of control and extract with scavenging activity (SA).

Calculation for Serial Dilution for Gallic Acid, Ascorbic Acid and Allium Sativum Ethanol Extract
All used stock solution with concentration of 3mg/ml, 10mL volumetric flask is used for dilution.
Use the dilution formula: C1V1 = C2V2
Whereas:
C1: Concentration of stock solution (3mg/ml)
V1: Volume of stock solution needed
C2: Final concentration after dilution
V2: Final volume of the solution (10mL)


The purpose of conducting this research project was to determine the phytochemical present inside the Allium sativum (garlic) ethanol extract, antibacterial and antioxidant properties. This involved a series of qualitative tests designed to identify different classes of compounds such as alkaloids, flavonoids, saponins, tannins etc. Identifying these phytochemicals is essential not only for understanding the bioactive potential of garlic. This initial screening set the stage for subsequent experiments focused on assessing the antibacterial and antioxidant activities of the extract, providing a holistic view of its therapeutic potential. Phytochemicals can be classified into two classes which are primary metabolites and secondary metabolites. Primary metabolites are essential in promoting primary growth and development of the plant whereas secondary metabolites do not have direct action on plant growth but protect plants from biotic factors like pathogens and microbes (Salam et al., 2023). In this research, the presence of primary metabolites which are carbohydrates and reducing sugars as well as secondary metabolites including alkaloids, flavonoids, terpenoids, glycosides, tannins, saponins and anthraquinones were determined. Maceration method was be chosen as the method for preparation of Allium sativum extract. The solvent that was used to retain active chemical constituents of A. sativum was ethanol. The ethanol extract was subjected to a series of phytochemical screening procedures. Furthermore, the garlic extract also showed a positive result in the saponins test, indicating their presence in the extract.
Saponins are major non-sulfur compounds found in garlic. These secondary metabolites are naturally occurring, heat-stable, amphiphilic glycosidic chemicals present in various parts of plants. Studies have proven that saponins in garlic can reduce blood lipids, inhibit oxidation and maintain vascular homeostasis in atherosclerotic rats, suggesting that garlic saponins are suitable for the prevention and treatment of atherosclerosis (AS) (Miao, et al. [15]). Additionally, saponins are stable during cooking processes and offer many therapeutic benefits, such as antitumor, antithrombotic and cholesterol-lowering effects. Their resilience and wide range of health benefits make saponins a valuable component in medicinal and dietary applications. Both primary metabolites, carbohydrates and reducing sugars were absent in the A. sativum extract. Others secondary metabolites such as flavonoids, glycosides, tannins and anthraquinones were also absent in all tested garlic extract. The assessment of antibacterial activity of Allium sativum was performed. The purpose of conducting antibacterial susceptibility testing is to evaluate its potential as a natural alternative to synthetic antibiotics. In recent decades, numerous natural substances have been identified as sources of effective antibacterial agents, with research increasingly focusing on plants as potential antimicrobial resources. The rise in antibiotic resistance and the need for sustainable alternatives to conventional antibiotics are what are driving this trend towards plant-based antimicrobials.
Through the in-vitro antibacterial activity assessment, the antibacterial effect of phytochemicals or any chemical constituent present in the plant extract can be studied by observing the zone of inhibition. This inhibition zone can help determine the ability of plant extract in inhibiting the growth of specific bacterial strains. The presence and diameter of the inhibition zone provide valuable insights into the potential of garlic extracts as effective antibacterial agents. In antibacterial activity study, two gram-positive bacteria (Bacillus subtilis and Staphylococcus epidermidis) in present research and one gram-negative bacteria (Escherichia coli) were used as tested bacterial strains. Gram-positive bacteria are characterized by a single, thick peptidoglycan cell wall. In contrast, gram-negative bacteria possess a thinner peptidoglycan cell wall along with an outer membrane that contains lipopolysaccharides. Bacillus subtilis, also called as hay bacillus is a rod-shaped, gram- positive bacterium in family of Bacillaceae. B. subtilis and other related Bacillus species are extensively used in biotechnology due to it offering several advantages which include absence of toxic byproducts and high production yields that are efficiently secreted. Staphylococcus epidermidis is another gram-positive bacterium used in this study. S. epidermidis is a cocci-shaped bacterium and belongs to the genus Staphylococcus. This bacterium is part of the normal human skin flora but can become pathogenic, particularly in immune compromised individuals or those with implanted medical devices.
It has a reputation for growing biofilms on surfaces, which increases its resistance to several medicines and makes the treatment more difficult. Escherichia coli is a rod-shaped gram- negative bacterium that derived from family Enterobacteriaceae. E. coli mostly can be found in the human gastrointestinal tract, and it is not pathogenic in this setting. While most strains of E. Coli are not harmful, some can result in uncomplicated cystitis and various other extraintestinal diseases, such as pneumonia, bacteremia, and abdominal infections like spontaneous bacterial peritonitis (Sykes, et al. [16]). Ciprofloxacin is an antibiotic agent in the fluoroquinolone class, and it was used as positive control in this study. The results showed zones of inhibition against all tested bacterial strains with ciprofloxacin. Based on the results, negative results were obtained in all tested bacteria strains by using Allium sativum ethanol extract. There were no zones of inhibition observed in all Mullen- Hinton agar in all nine petri plates. The garlic extract was prepared in two concentrations, 50 mg/mL and 100 mg/mL, diluted with sterile distilled water. The use of sterile distilled water was essential to prevent the presence of pathogenic bacteria that could compromise the integrity of the garlic extract and potentially affect the accuracy of the results. For the antibacterial tests, Mullen-Hinton agar was chosen as the growth medium. This standard agar is widely employed in research due to its ability to support the growth of various microorganisms without inhibiting their development.
One of the factors influencing the antibacterial activity of the garlic
extract against the tested bacterial strains was its relatively low
phytochemical content. As a result, the prepared extract did not exhibit
sufficient potency to inhibit the growth of the bacteria tested,
leading to no observable response. Total phenolic content (TPC) is the
method utilized to identify the phenolic content in the plant extract.
The phenolic composition that can be found in plant extract possesses
redox properties, that enable them to act as antioxidant agent by
neutralizing free radicals and preventing oxidative stress (Johari, et
al. [17]). Folin-Ciocalteu assay is used in this study for TPC testing. It
is one of the most famous assays used in phenolic analysis. This assay
is based on the redox reaction between phenolic compounds and
the Folin–Ciocalteu reagent, which contains phosphomolybdate and
phosphor-tungstate in a strong acid solution. Then, it’s reduced to
produce blue coloration when mixed with samples that contain phenolic
compounds (Pérez, et al. [18]). The blue color complex produced
will have maximum absorbance at wavelength of 760nm which can
be measured using UV spectrophotometer. Gallic acid acts as the standard
for TPC testing due to its well- characterized phenolic structure
and potent antioxidant properties. The graph of absorbance against
concentration of gallic acid standard that plotted shows linear line
which indicates that they were directly proportional to each other.
TPC present in the Allium sativum ethanol extract was defined in
term of GAE and can be calculated by using the equation that generated
from the graph (y = 0.1375x + 0.155 and R2= 0.9707). The TPC of
the gallic extract can be calculated by using formula of  TPC of extract was found to be 19.39 mg GAE/g. 2,2-diphenyl-1-picrylhydrazyl
(DPPH) assay was used to determine the antioxidant
activity of the Allium sativum ethanol extract by measuring the percentage
of scavenging activity of DPPH present in the tested solvent
extract. It utilizes free radicals to assess the ability of substances to
act as hydrogen donors or free-radical scavengers (FRS). This technique
involves the reduction of DPPH, a stable free radical, which
serves as the basis for measuring the antioxidant potential of the tested
samples. The free-radical DPPH interacts with an odd electron to
yield a strong absorbance at 517 nm (Baliyan, et al. [19]). When various
concentrations of plant extracts were mixed with DPPH, a purple
color forms due to the presence of DPPH radicals. As the stable
DPPH radical undergoes oxidation, the purple color gradually fades
to yellow. This color change indicates the occurrence of DPPH radical
scavenging, which can be quantified by measuring the absorbance at
517 nm using a spectrophotometer. Ascorbic acid acts as standard for
the DPPH assay due to its strong antioxidant properties and ability
to scavenge free radicals. It has high reducing power which can effectively
reduce the DPPH radical to its non-radical form, resulting in a
color change from purple to yellow, which can be quantified by measuring
the absorbance at 517 nm.
TPC of extract was found to be 19.39 mg GAE/g. 2,2-diphenyl-1-picrylhydrazyl
(DPPH) assay was used to determine the antioxidant
activity of the Allium sativum ethanol extract by measuring the percentage
of scavenging activity of DPPH present in the tested solvent
extract. It utilizes free radicals to assess the ability of substances to
act as hydrogen donors or free-radical scavengers (FRS). This technique
involves the reduction of DPPH, a stable free radical, which
serves as the basis for measuring the antioxidant potential of the tested
samples. The free-radical DPPH interacts with an odd electron to
yield a strong absorbance at 517 nm (Baliyan, et al. [19]). When various
concentrations of plant extracts were mixed with DPPH, a purple
color forms due to the presence of DPPH radicals. As the stable
DPPH radical undergoes oxidation, the purple color gradually fades
to yellow. This color change indicates the occurrence of DPPH radical
scavenging, which can be quantified by measuring the absorbance at
517 nm using a spectrophotometer. Ascorbic acid acts as standard for
the DPPH assay due to its strong antioxidant properties and ability
to scavenge free radicals. It has high reducing power which can effectively
reduce the DPPH radical to its non-radical form, resulting in a
color change from purple to yellow, which can be quantified by measuring
the absorbance at 517 nm.
This reduction is directly proportional to the concentration of ascorbic acid. The scavenging activity increases with the increases in concentration, this allows for plotting of a standard curve as shown in the text. The scavenging activity of ascorbic acid standard and Allium sativum ethanol extract can be calculated via the following equation:
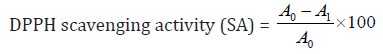
Whereas A0: absorbance of control; A1: absorbance of sample.
Through this equation, the percentage of scavenging activity of each concentration of the ascorbic acid standard and A. sativum ethanol extract was determined. The concentration of both ascorbic acid standard and A. sativum ethanol extracts were prepared in 3.0mg/ ml, 2.4mg/ml, 1.8mg/ml, 1.2mg/ml, 0.6mg/ml and 0.3mg/ml. The scavenging activity of ascorbic acid standard ranged from 59.92% to 83.86%, whereas for A. sativum ethanol extract, the range of scavenging activity is from 34.35% to 67.34%. When the concentration of ascorbic acid standard and A. sativum ethanol extract increase, the absorbance value obtained because will decrease due to the increased number of DPPH radical scavenged by the high concentration of ascorbic acid and the phytochemicals that extracted from A. sativum extract. By comparing the scavenging activity of ascorbic acid and A. sativum ethanol extract, it was found that scavenging activity of ascorbic acid is much higher compared to A. sativum ethanol extract that in same concentration.
IC50 value refers to the concentration that is required by the sample to result in inhibition of 50% of the free radical activity (Reviana, et al. [20]). The lower the IC50 value, the higher the free radical scavenging activity. The IC50 of the ascorbic acid standard and A. sativum ethanol extract were found to be 18.35 mg/ml and 1.41 mg/ml respectively. This indicates that the garlic extract possesses stronger antioxidant properties and can effectively act as an antioxidant agent to combat free radicals and oxidative damage (Ali, et al. [21-44]).
From the conducted research, Allium sativum can be concluded to exhibit several pharmacological actions due to the presence of phytochemicals which include alkaloids, terpenoids and saponins in the A. Sativum ethanol extract. A. Sativum ethanol extract also possesses strong antioxidant properties which can neutralize free radicals that may harm our cells and tissues. Hence, A. Sativum can protect our body from any oxidative stress. A. Sativum is believed to exhibit antioxidant action due to some active chemical constituents like saponins and terpenoids. Allium Sativum also proved to have antibacterial activity towards several bacterial strains although negative results were obtained in this study conducted. This may be due to several reasons which include insufficient concentration of active chemical compounds that present in the garlic extract. Even though concentrations of 50 mg/mL and 100 mg/mL were used, they might not have been sufficient to inhibit or kill the tested bacterial strains. Besides, this may be because of the extraction method that has been used. The maceration method may not efficiently extract the active compounds that can exhibit antibacterial properties. To address these issues, further studies could explore different extraction methods, higher concentrations of the extract, and other bacterial strains.
Additionally, optimizing experimental conditions and using freshly prepared extracts with known stability could help better evaluate the antibacterial potential of Allium sativum. Long-term research can be conducted with the purpose of further study A. sativum to gain better insights on other pharmacological actions that can be exhibited by this plant and using different extraction methods such as Soxhlet extraction or using different solvent for extraction like methanol to preserve the maximum phytochemicals that present in the Allium sativum.
The authors are much grateful to the Faculty of Pharmacy, AIMST University, Malaysia for funding and providing all facilities to carry out this research project.
Authors declare that there is no conflict of interests.
Thanks to science officers, Ms. Amalina and Ms. Jaya of MDL 4 laboratory.


