Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ogunsina OI1,2, Omolayo A3 and Olusola AO1*
Received: July 04, 2024; Published: July 24, 2024
*Corresponding author: Olusola AO, Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Nigeria
DOI: 10.26717/BJSTR.2024.57.009052
Croton hirtus L'Hér belongs to the family Euphorbiaceae, which is one of the most important plants used in folk medicine. The study was carried out to evaluate the protectant effect of the stem powder against stored bean weevils, Callosobruchus maculatus. Protective activities of C. hirtus stem powder as well as Permethrin 0.05g standard insecticidal powder were evaluated on cowpeas infested with Callosobruchus maculatus at different concentrations of 0.2, 0.4, 0.6, 1.0, and 2.0 g powder per 50 cowpea seeds and incubated at ambient conditions for up to 50 days. Data was collected on level of oviposition at 15 days after treatment (DAT), adult emergence, cowpea damage, and cowpea weight loss at 50 DAT were obtained. Increasing protectant activity against bruchids was observed with increased concentrations of C. hirtus stem powder with reductions in oviposition ranging from 34.3 to 18.5 and adult emergence ranging from (97.17-50.43 %), compared to permethrin-positive control 0.05g (19.3% and 55.52%). Seed weight loss of cowpea in a concentration-dependent manner ranged from 16.6% to 6.1%, respectively. However, the percent protectant ability of C. hirtus powder from 0.2–2.0 g/50 seeds (20.7–37.3%) increased with an increase in concentration, compared with permethrin at 50%. The phytochemical screening revealed secondary metabolites such as tannin, saponin, terpenoids, and cardiac glycosides. The results indicate the potential of C. hirtus stem powder as a stored cowpea protectant against Callosobruchus maculatus.
Keywords: Cowpea; Croton Hirtus; Callosobruchus maculatus
Cowpea (Vigna unguiculata) is a major food legume growing in Africa's tropical savannah zones and other tropical countries around the world [1]. Cowpea is critical to the livelihoods of millions of relatively impoverished people in tropical, less developed, and developing countries. Its popularity stems from the fact that rural families profit from it in different ways, including food, animal feed, and cash, as well as spill over advantages to their farmlands. It serves an important subsistence role in the diets of many households around the world, providing cheap and nutritious food in areas where cereals are scarce [2]. Despite this, many producers believe its cultivation to be a dangerous investment because of the various pest problems associated with it [2]. In most cowpea-producing countries, insect damage is the most significant constraint on cowpea grain production. Cowpea production is hampered by insect pests and disease infestations, which cause economic losses [3]. The cowpea weevil is a worldwide field-to-store pest that is the primary post-harvest pest of cowpea in the tropics.
According to [1], the main problem that farmers face is the conservation of cowpea crops because of two bruchid species, Callosobruchus maculatus and Bruchidius atrolineatus, which can destroy 80 to 100% of grains in 2 to 3 months after harvest, and virtually all of the grain may have holes by 6 months. Cowpea weevils, notably Callosobruchus maculatus, cause damage to dried cowpeas and other stored seeds after harvest. The weevil favours dried cowpeas but will attack other stored beans and peas [4]. Insect pest infestation causes significant quantitative and qualitative losses, as evidenced by seed perforation and decreases in seed weight, market value, and germination potential. Consumers have a strong antipathy to weevil-damaged grain, but it can still be used as seed, though the germination rate may be lowered. To avoid major losses during storage, various approaches and control mechanisms have been devised, and more are being developed. Cowpea seed storage pest management is mainly reliant on the use of chemical insecticides [5]. However, due to financial and technological constraints, the majority of small-scale farmers have not implemented these new practices.
Insecticides are also harmful to the environment, humans, livestock, and non-targeted creatures. Such chemical overuse on cowpea, along with low yields, has resulted in an extensive search for pest control options with more acceptable techniques for controlling insect pests of cowpea in storage with little or no insecticide input, or bio-intensive integrated pest management (lPM). Research efforts to ensure sustainable food production for the growing human population have revealed that produce loss is one of the most serious issues confronting food production in sub-Saharan African countries [6]. Harvesting, handling, and storage procedures must be matched by a fast increase in crop yield and a necessity for sustainable production to reduce post-harvest losses. Among the options is the use of resistant cowpea varieties to weevil attack [1]. However, the use of resistant cultivars is mostly ineffective since large rural farmers lack access to them. Croton (Euphorbiaceae) has over 1200 species, the majority of which are found in tropical and subtropical regions of both hemispheres [7].
Several members of the genus are widely used in folk medicine to treat a variety of illnesses, including inflammatory conditions, pain, diabetes, hypertension, malaria, gastrointestinal disturbances, and ulcers [8,9]. Croton hirtus L. also known as C. glandulous, is an erect shrub whose chemical composition has been studied in numerous studies. It is the second-biggest genus in the Euphorbiaceae family, with several species utilized in traditional medicine throughout the world. The trans-terpenoids dehydrocrotonin, derived from Croton species, have been shown to exhibit hypoglycemic, hypolipidemic, anti-estrogenic, anti-tumor, and trypanocidal properties [10].
Callosobruchus maculatus is the most frequent and damaging member of the Bruchidae family. The inner carina of the hind femur of this species is smooth, and the inner tooth is usually longer than the outer tooth. The adult pronotum is covered in black cuticle and golden setae, except for the basal median gibbosites, which extend well beyond the posterior edge, which is covered in white scale-like setae. Their eyes are highly emarginated, prominent, and bulbous. These species' male genitalia are unique, median lobed, and have two longitudinal sclerotized denticulate regions around the middle [11]. Callosobruchus maculatus (Fabricius) is a worldwide and economically significant storage pest that is found in tropical and subtropical climates. Cowpea, Vigna unguiculata (L.) Walp, is one of the five most important legumes in the tropics, providing protein to the majority of the region's inhabitants as well as nitrogen to the soils [12].
Drugs and Chemical
Rambo (permethrin, 0.60% w/w) insect powder.
Collection and Preparation of Cowpeas
Cowpeas seeds (Ife brown variety) used in this study were purchased from the local Okada market and handpicked to remove infested seeds and other debris. Seeds that showed no visible signs of weevil attack were separated, bulked, and transferred into a transparent plastic container (350 mL) covered with fine mesh for aeration and incubated at ambient conditions (25–30 °C, 70–80% r.h.) for 3 weeks to remove all evidence of prior infestation by the cowpea weevil before bioassay.
Preparation of Plant Materials
Fresh stems of Croton hirtus were obtained from different locations at the Crown Estate of Igbinedion University campus, authenticated, and stored in the pharmacognosy department herbarium with voucher number IUO/19/290. The materials were air-dried under laboratory conditions (temperature range 25–30 °C; 70–80% r.h.), ground to a fine powder, and wrapped in cellophane bags until needed.
Callosobruchus maculatus Culture
The method for rearing the experimental insects followed the procedure described by [13]. Adult C. Maculatus were originally obtained from infested samples of cowpea bought from Okada Market, Edo State. They were reared on uninfected cowpea seeds inside a transparent jar at a temperature of 28 ± 2°C and 70% relative humidity. A total of 10 pairs of newly emerged 20-day-old adults were introduced into the rearing jar containing 100 pieces of cowpea grains. The jar was covered with a jar cover to prevent contamination of the seeds and the escape of the bruchids. A maximum of 15 days was allowed for mating and oviposition. The parent bruchids were removed afterward, and the seeds containing the eggs were left in the rearing jars, which were also covered as described above. The rearing was done at the above-mentioned temperature and relative humidity after collection for F1 generations to allow for the multiplication of the weevil for the experiment [14]. The subsequent progenies emerging from the stock were used as parental generations for the experiment [15].
Fifty cowpea seeds, after being weighed to determine their initial weight, were placed in each experimental jar for the infestation. All the cowpea varieties were infested with C. maculatus obtained from the stock culture. A total of five pairs of newly emerged 15-day-old adults, males and females, were selected and introduced into each experimental transparent jar containing the 50 cowpea seeds. The insects were allowed 15 days to mate and lay eggs, after which they were removed from the jars with the help of a fine mesh sieve. The experimental transparent jars were covered with perforated lids. Each experimental jar was tightly closed and well labelled. They were placed under laboratory conditions maintained at a constant temperature of 28 ± 2°C and 50–70% relative humidity.
Phytochemical Screening
Phytochemical screening is a process in which the extraction, identification, and screening of phytochemicals are done in the crude sample extract. The stem powder of Croton hirtus was subjected to qualitative tests for the presence or absence of various phytochemical components present in the stem powder, namely alkaloids, flavonoids, polyphenols, tannins, saponins, terpenoids, and steroidal compounds. The phytochemical screening was carried out by chemical testing according to standard procedures [16].
Test for Saponins: Foam Test: 2 ml of sample powder was taken in a test tube and dissolved in 15 ml of distilled water. Then the stoppered test tube was shaken vigorously for 15 minutes. Frothing Test: 0.5 g of sample powder was diluted with 20 ml of distilled water and shaken for 15 minutes. In both cases, the formation and retention of 1cm of foam or honeycomb froth for 15 minutes is considered to indicate the presence of saponin in plant extract.
Test for Terpenoids: Salkowski Test: Two milliliters of chloroform and three milliliters of concentrated sulfuric acid were carefully added to one milliliter of sample powder. A reddish-brown coloration will signify the presence of terpenoids in the sample.
Test for Cyanogenic Glycosides: Place a small amount of powder drug in each of the 3 test tubes and label A, B, and C. Mix the powder in A and B with a little water, and insert a freshly prepared piece of sodium picrate paper in each of the test tubes. Stopper the test tubes immediately and place test tube B in a boiling water bath for 5 minutes. Keep tubes A and C at room temperature. Changes in change indicate cyanogenic glycosides in the sample.
Test for Tannins: Ferric Chloride Test: One milliliter of sample powder was taken into a test tube, and 10% of ferric chloride was added to it, containing the sample, and stirred well. The formation of a bluish-black precipitate shows the presence of tannins in the sample.
Test for Flavonoids: To 2 ml of Croton hirtus stem powder, add a few drops of lead acetate. A milky precipitate indicates the presence of flavonoids. Also, to 1 ml of the sample in a test tube, add 1 ml of dilute ammonia solution. A yellow coloration indicates the presence of flavonoids.
Test for Alkaloids: Wagner’s Test: 5 milliliters of sample powder were measured and placed in a test tube. 10% hydrochloric acid was added to a test tube containing the sample and filtered using filter paper. The filtrate was treated with Wagner reagents, and the formation of a brown-reddish precipitate indicates the presence of alkaloids. Wagner reagent contains 2 grams of iodine and 6 grams of potassium iodide, which were dissolved in 100 ml of distilled water.
Test for Anthraquinones (Borntrangers reaction): 5 milliliters of the sample powder with chloroform (2 x 5 ml) in a separatory funnel. Separate the organic layer into another separatory funnel and add 5 ml of a 10% ammonia solution. Shake again and observe the formation of a pink, red, or violet colour in the ammoniacal (lower) layer, which indicates the presence of free anthraquinones.
Test for Cardiac Glycoside: Add 2 ml of chloroform to 2 ml of extract in a test tube and put in an ice bath in a fume cupboard. Tilt the tube and carefully add 1-2 ml of H2SO4 to form a lower layer. A reddish-brown colour at the interface indicates the presence of a steroidal ring (the aglycone portion of the cardiac glycoside).
Cowpea Bioassay with Croton hirtus Stem Powder
Cowpea bioassay with Croton hirtus stem powder. Different weights (0.2, 0.4, 0.6, 1.0, and 2.0 g) of the Croton hirtus stem powder were added to 50 cowpea seeds separately in 300-mL transparent plastic cups and firmly covered. The powder was spread over the seeds by shaking the cup. The negative control treatments did not have any powder. The positive control treatment contained 0.05 g of permethrin and 0.60% w/w insecticide powder. Newly emerged C. maculatus (5 males and 5 females) were introduced into each transparent jar, covered firmly, and locked in the laboratory at ambient conditions. Each treatment was replicated three times. At 15 days after treatment (DAT), all the insects were removed from the cups to prevent them from mixing with the first-generation (F1) offspring as they later emerged, and an egg count was established. Once the emergence of adult insects started, the test and control samples were checked every 3 days’ interval, the number of newly emerged adults determined, and then removed from the cups. This periodic removal of F1 offspring continued up to 50 DAT, when there was no evidence of further emergence. At this stage, other parameters such as adult emergence, seed damage, and seed weight loss relating to the condition of the cowpeas were also recorded. The means of all determinations (± SEM) were recorded.
Data Collection: Parameters measured included oviposition, adult developmental period, progeny emergence grain damage and grain weight loss.
Oviposition: The number of eggs laid on the seeds of each sample was counted separately following the method described by [17]. They were recorded for each treatment two weeks after the infestation. The grains were then re-incubated until the emergence of the F1 progeny.
Adult Emergence: The various treatments were examined at three (3) days interval for the proportions of adults that emerged from the number of eggs laid on the seeds, including hatched and unhatched, following the method of [18]. The emerged adults were removed from each sample by sieving with a fine-meshed sieve. F1 progeny assessment started at the time of adult emergence by counting all emerged insects, both live and dead ones.
Grain Damage: To determine grain damage rate, samples of 150 grains were taken randomly from each treatment. The number of damaged (grains with characteristic holes) and undamaged grains were counted and the rate calculated using the formula;
Where; Nd = Total Number of damaged seeds and
Nu = Total Number of whole seeds [19].
Seeds Weight Loss: Weight loss caused by maculatus infestation was assessed on the grains of all varieties after the 50days of storage. The damaged and undamaged grains, after being sorted and counted, were weighed, and the weight loss assessment was computed using the following formula:
Where; Wi = Initial Weight, Wf = Final Weight [20].
Data Analysis
Results were expressed as a mean ± standard error of mean (S.E.M). Statistical analysis of the data was performed using GraphPad Prism Software 8 and was carried out using one ways ANOVA test followed by Dument. Significance difference was set at P > 0.05 level. Percentage damage (PD) and weevil Perforation Index (WPI) was calculated according to the methods of [21].
Preliminary Phytochemical Screening
The phytochemical screening of Croton hirtus stem powder showed that saponin, terpenoids (triterpenoids), tannins, cardiac glycosides, and cyanogenic glycosides were present. Saponin, terpenoids, cyanogenic glycosides, tannins, and cardiac glycosides occurred in high amounts, while flavonoids, alkaloids, steroids, and anthraquinones were absent (Table 1).
Note: Key: +++ (Most Present); ++ (Moderately Present); ‒– (Absent)
Results of Cowpea Bioassays:
Oviposition and Adult Emergence: The results showed that the mean number of eggs laid on the cowpea seeds were significantly (p < 0.05) affected by the varied concentration. With regard to oviposition effect, the result showed that in cowpeas treated with Croton hirtus stem powder, 34.3, 31.1, 27.4, 23.25, and 18.5 respectively for 0.2-2g concertation, while the positive and negative control were 19.3, and 54.33 respectively and results were recorded within 15 DAT as showed in Table 2. This shows that Croton hirtus stem powder has ability to protect cowpea seeds from weevil infestation. Figure 1 shows the effect of cowpea varieties on the mean adult emergence of maculatus (F.) from the cowpea seed during the treatment period 50 DAT. There is significantly (p < 0.05) higher percentage of adult emergence with respect to concentration of the stem powder, (97.17%. 95.36%, 66.89%, 54.45% and 50.43%) respectively. In contrast, permethrin standard insecticidal powder had the lowest number of adult insect emergences.
Table 2: Effect of Croton hirtus stem powder on adult emergence of C. maculatus on cowpea at 50 DAT.
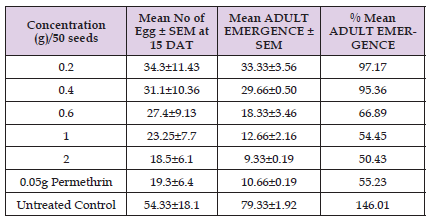
Note: Each value is the mean of three replicate
Cowpea Weight Loss: The losses in weight of cowpea seeds due to infestation by the Callosobruchus maculatus are presented in Table 3. Weight loss was found to be significantly different (p < 0.05) among the varied concentration. When seeds weight loss was converted into percentages, significant differences (p < 0.05) were still observed among the different concentration. Percentage seeds weight loss were highest in (0.2-0.4) g. Cowpea seeds treated with Croton hirtus stem powder had a slight lower grain weight loss of 16.1%, 15.4%, 8.8%,7.2% and 6.2% respectively corresponding to concentration of 0.2-2g respectively, as against 5.9% of positive control.
Table 3: Effect of Croton hirtus stem powder on seed weight loss of C. maculatus on cowpea at 50 DAT.
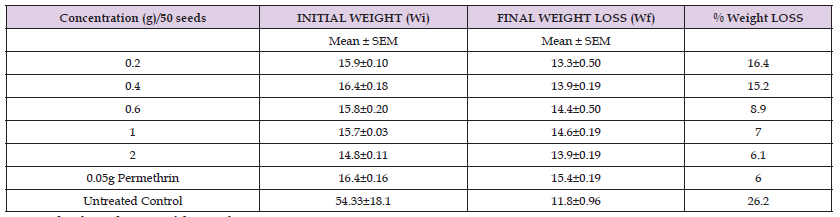
Note: Each value is the mean of three replicate
Cowpea Damage: The effects of Croton hirtus stem powder on cowpea seed damage due to infestation by the cowpea weevil, Callosobruchus maculatus is presented in Table 4. The percentage cowpea seed damage in the Croton hirtus treatments were 79.3%, 69.3 %, 63.3%, 44.6% and 34.6% of (0.2,0.4,0.6,1.0 and 2.0) g stem powder respectively as against 20.7% of 0.05g Permethrin and 94.6% negative control. Percent damage was highest in Croton hirtus2g stem powder, 2.0g concentration gave a higher protection against damage. The Weevil Perforation Index which indicates the ability of the Croton hirtus stem powder in protecting the cowpea seeds ranged from 79.3% to 62.7% (0.2-2.0) g as compared to the control (50% for positive control and 82.1% for negative control). values of this index above 50 indicate negative protectant ability. The effectiveness of the Croton hirtus as protectant of stored cowpea against damage by C. maculatus represented as percent protectant ability showed that stem powder concentrations of 0.2-2g and resulted in 20.7 -37.3% ability as compare to the control (50% for the positive control). The average percent protectant ability is represented graphically in Figure 2.
Note: Each value is the mean of three replicate. *Weevil Perforation Index (WPI) above 50 is an indication of negative protectant ability.
The results of the qualitative phytochemical analysis of Croton hirtus stem powder are presented in Table 1. The presence of saponin, tannins, terpenes, cyanogenic glycosides and cardiac glycosides in the phytochemical profile of the Croton hirtus stem powder was consistent with the findings of [22], who found similar phytochemicals in Croton species plants. Though secondary plant metabolites produced by plants are for defensive purposes, some of these compounds have been identified and used as substances that are toxic to insects. It is also important to note that the absence or presence of these bioactive compound groups depends on the polarity of the solvent used as reported by [23]. The persistent and high concentration of foam in the sample indicates that Croton hirtus stem are rich in saponins. The presence of saponins in the Croton hirtus stem of the plant is used to defend different herbivores and improves its use as insecticide for different life stages of pests. Plants rich in saponins defend herbivores from feeding on the host plant and lose their movement, thus causing pest mortality due to the high toxicity of saponins. The saponin also affects the insect pest indirectly by making different bonds with multiple digestive enzymes, which damages the mucus line of a number of cells in the digestive system [24].
The intense color change of sodium picrate test paper shows an indication of the presence of cyanogenic glycosides in the sample, like many other plant natural products, cyanogenic glycosides serve as defense agents against herbivores by releasing toxic hydrogen cyanide after tissue damage [25]. Varying degrees of success have been recorded by many farmers in the tropics in the use of botanicals to protect their legumes [26]. Numerous plant parts, including Croton species, appear to have a promising level of control over pests. Croton hirtus stems are cultivated widely in the tropics and therefore offer opportunity for developing their products as alternatives to hazardous pesticides to protect stored cowpeas from pest damage [26] have discussed some advantages of using plant parts as grain protectants, and may have very low toxicity to mammals since some of the plants are used as food flavouring but they are cost effective and their application is easy. Developmental period of C. maculatus was recorded as the time taken to develop from egg laying to adult emergence.
The extent of this period actually depended on several factors. The nature of the substrate, temperature, and relative humidity are some of the factors that might affect the developmental period of C. maculatus [27]. When C. maculatus was reared within the confinement of the required temperature and relative humidity, the number of days to complete development by the insect was determined by a preferred host. In a situation where temperature and relative humidity were taken into consideration, host preference was determined based on the egg-adult developmental time of the beetle [27]. From the data collected in this study, Croton hirtus stem powder concentration negatively influenced the developmental period of the beetle by reducing the number of adults to emerge after oviposition at higher concentration of 2.0 g, whereas the number of adult emergences in negative control was high compared to the positive control. This means that the highest mean number of progeny emergences was also observed with respect to the concentration of Croton hirtus stem powder. The results are in correlation with the work of [17].
As documented by [15] and [28], the extent of damage and subsequent weight loss of cowpea grains highly depend on the number of adult emergences of C. maculatus on the cowpea. Thus, the higher the number of F1 progeny emergences on the cowpea, the higher the damage and weight loss of the cowpea. Damage caused by C. maculatus from this study followed the pattern of emergences with respect to the concentration of the croton hirtus used. The results of the Croton hirtus stem powder treated on cowpea seeds to evaluate damage due to infestation by Callosobruchus maculatus in this study showed that the effectiveness of the stem powder was relatively ideal. The results showed that Croton hirtus stem powder proved effective in reducing damage to seeds, lowering the weevil perforation index, and increasing protectant ability. Damage to cowpea seeds in this study was very low due to the protection of seeds by the Croton hirtus stem powder. The percentage damage to seed ranged between 79.3% and 34.6% with respect to ascending order of concentration (0.2 to 2.0) g respectively, while the percentage damage to positive and negative control was 20.7% and 94.6% respectively.
Percentage seed damage in ascending order of concentration of Croton hirtus stem powder treatments showed higher effectiveness at preventing damage. The percentage damage in untreated (negative control) seeds in this study was about 94.6%, this value is relatively higher than that of the stem powder treated and positive control (0.05g permethrin) experiments. According to [28], the loss in mass of cowpea positively correlates with the number of emerged insects. This loss is an important parameter to measure both from an economic point of view and as an indicator of a cowpea variety’s resistance to C. maculatus. This is explained by the fact that the feeding activities of the insect lead to perforations (damage). It is therefore this damage that brings about the reduction in weight loss experienced by the cowpea. The weevil perforation index (WPI), which indicates the protectant ability, was significantly lower in the Croton hirtus stem powder and positive treated experiments than in the negative treated control; the value recorded in the negative control was higher than 50.
Meanwhile, values above 50 is usually an indication of negative protectant ability [29]. This study recorded a value of 82.1% in the negative control compared to 50% recorded in the positive control treatments, the higher values in the Croton hirtus stem powder treatment may be a low concentration of the tested sample of (0.2 to 0.6) g of Croton hirtus stem powder but (1.0 to 2.0) g of Croton hirtus stem powder, showed a slight value above 50%, which is an indication of its protectant ability of the Croton hirtus stem powder. The effectiveness of Croton hirtus stem powder as a cowpea seed protectant against C. maculatus manifested by percentage protectant ability indicated that the levels of protection were corresponded to the concentration of the dosage of Croton hirtus stem powder in the treated experiment compared to the negative control. Seed protection was highest in positive control treatments with 0.5g permethrin where an average of 50% protection was achieved, whereas 31.6% and 37.3% protection were achieved in (1.0 to 2.0) g concentrations of Croton hirtus stem powder. The effectiveness could be due to coating of the seed by the tested sample [29].
The results obtained in this study revealed that Croton hirtus stem powder has a potential in protecting cowpea seeds from C. maculatus damage. Croton hirtus stem powder may therefore be incorporated and adopted for the control of pests to further reduce the use of synthetic pesticides.
We appreciate the efforts and technical skills of Miss Okeke Chidera (Technologist, Department of Pharmacognosy, College of Pharmacy, Igbinedion University, Okada) and Miss Adaramola Faith (Laboratory Unit, Department of Biochemistry), Adekunle Ajasin University, Akungba Akoko, Nigeria.
There was no declaration of interest and no conflict among the authors in presenting this article for publication.


