Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sevil Mehraliyeva*
Received: July 10, 2024; Published: July 22, 2024
*Corresponding author: Sevil Mehraliyeva, Azerbaijan Medical University, Department of Pharmaceutical Technology and Management, Azerbaijan
DOI: 10.26717/BJSTR.2024.57.009044
Objectives: In the research work, a complex of biologically active substances obtained from licorice (Glycyrrhiza
glabra), sophora (Sophora japonica), clover (Trifolium pratenze), and calendula officinalis plants; Propolis
alcoholic extract; Chitosan; Lavender essential oil; Glutamic acid; Zinc oxide; Sorbitol; Twin-80; Ethyl alcohol;
Nipagin; Nipazole; Purified water was used.
Materials and Methods: The qualitative reaction of “Glysotrical” gel prepared in the pharmaceutical technology
laboratory of AMU was performed by chromatography on a thin layer, determination of pH by potentiometric
method. The determination of rutin and glycyrrhizic acid in the studied gel was carried out by the High-
Performance Liquid Chromatography (HPLC) method (on the Agilent 1100 American chromatograph at 360
and 260 nm wavelengths). The rheological properties of Glysotrical gel were studied in the “Reotest-2” device.
Inspections related to microbiological cleanliness were carried out in accordance with the requirements of
the XIII State Pharmacopoeia. The effect of “Glysotrical” gel preparation was evident during the treatment of
chemical and thermal burns created in chinchilla rabbits.
Results: The rheological properties of “Glysotrical” have been described. It was revealed that the shear strength
(458 N/m2 and 355 N/m2) of the eel preparation at 20°C and 40°C is lower and the value of the dynamic viscosity
(912cPz and 602cPz) is higher than pure chitosan gel. As a result of rheological studies, it was found that no
visible mechanical-structural changes occur in the gel system at 20-40°C. This proves that it remains stable
in those temperature regimes. This makes it possible to use the prepared medicinal form in different climatic
conditions (20-40°C) without breaking its stability. Different results were observed in 60°C. Accordingly, it was
observed that the value of slip resistance and effective viscosity decreased at 20 and 40°C. As a result of the
conducted research, it was determined that the ingredients included in the composition of the gel preparation
actively participate in the formation of its dominant mechanical-structural system. Among them, the most
important factor affecting the stability of the dispersed system is twin-80. So, during the rheological studies
conducted at 60º C temperature, the weakening of the mechanical dominance of the system results in a very
low decrease of its dynamic viscosity (287sPz) and shear stress value (212 Н/m2). The gradual decrease in
the solubility of Tween-80 at a temperature of 60°C weakens the mechanical dominance of the system and
weakens the reciprocity between the particles of the dispersed phase. This also causes a decrease in the value of
acceleration and dynamic performance.
Bae systems playained results, it can be noted that the inclusion of Tween-80 in the composition of the prepared
gel system plays an important role in the change of its biomechanical properties, as well as in obtaining a stable
mechanical structural form. The beneficial effect of “Glysotrical” was evident during the treatment of chemical
and thermal burns in Chinchilla rabbits. As a result of the comparative studies conducted with “Glysotrical” gel,
it was found that during the treatment of chemical burn with “Glysotrical” gel, the healing speed of the burn was
53.73%, while the healing speed with calendula ointment was 21.92%. During the treatment of the thermal burn
with “Glysotrical” gel, the wound healing rate was 50.2%, while the healing rate with calendula ointment was
19.96%. As a result of operations carried out for 24 months during storage of the studied gel, it was clear that it
is possible to ensure the stability of the gel when stored in a dark place at temperatures of 10 and 22°C. The gel
should be released in tubes of 15g and 30g, used for medical purposes (TС-64-7-678-90) or orange glass vials.
The preparation should be stored in a dry place, protected from light, at a temperature of 10-22°C. The storage
period is 2 years.
Keywords: Glysotrical Gel; Rutin; Glycyrrhizic Acid; Rheological Properties; Anti-Inflammatory Effect; Stability
Abbreviations: HPLC: High-Performance Liquid Chromatography; AD: Atopic Dermatitis; NOS: Nitric Oxide Synthase; NO: Nitric Oxide; VAS: Visual Analog Scale; RCE: Red Clover Extract; LPS: Lipopolysaccharide
One of the important problems facing pharmaceutical science is to develop the technology of new drugs of natural origin in the treatment of dermatological diseases. Despite the increase in the number of Phyto preparations for the treatment of skin diseases (mycosis, psoriasis, eczema, burns, etc.), the increase in demand for these preparations creates the basis for conducting research in the direction of creating new Phyto remedies. Considering the mentioned relevance, a new gel medicinal form was developed based on plant raw materials from the flora of Azerbaijan, as well as some natural remedies of animal origin (chitosan, propolis) and auxiliary substances. The pharmacological and biopharmaceutical properties of the proposed “Glysotrical” gel have been studied by us [1-5]. It is known that Licorice (Glycyrrhiza glabra L., Fabaceae) is a perennial plant famous for its sweet-tasting root. It contains many bioactive natural products. Glycyrrhizin, the sweet principle of licorice root, is a triterpene-type saponin with antiviral, anti-inflammatory, antitumor, and antimicrobial properties. In addition to glycyrrhizin, phenolic components such as the chalcone is liquiritigenin and the is flavonoid glabridin are also important for the observed biological activity of licorice root. G. glabra has traditionally been used to promote wound healing. Licorice root extracts protect the skin from oxidative stress injuries, accelerate wound epithelization, improve wound site remodeling, and effectively reduce symptoms of Atopic Dermatitis (AD).
In addition, isoliquiritigenin was also found to be beneficial for the treatment of AD-like skin lesions in mice, giving hope that it may be a potential therapeutic agent for the treatment of AD in humans. Glabridin has many properties that are potentially useful in cosmetic products. It acts as an antioxidant, estrogenic, anti-inflammatory and skin whitening agent. It exhibits skin depigmentation activity and is included in topical products specifically designed for this purpose [6- 8]. Dried flowers and buds of Japanese sophora (Sophora japonica, Huaihua in China, Flos Sophorae) and immature flower buds (sophora rice, Huaimi in China, Flos Sophorae immaturus) are used for bleeding hemorrhoids, hemositis, hemositis, hypertension and pyoderma. Flower buds have a bitter taste, have cooling effects, can remove heat and clear “fire”, and for these reasons are used in the treatment of hemorrhoids, hematochezia, hemoptysis and purulent skin diseases. The flower buds of S. japonica have anti-inflammatory, antioxidant, anti-diabetic, and anti-tumor activities (and interestingly, others have skin whitening properties as the buds and some of their components inhibit the auto-oxidation of tyrosinase and DOPA (dihydroxyphenylalanine). found that it has and protects human keratinocytes against UVB radiation [8]. UPLC-ESI-MS profiling of the flavonoid-rich fraction of G. glabra roots and S. japonica leaves resulted in the preliminary identification of 32 and 23 compounds, respectively.
In addition, the wound healing potential of topical preparations of each fraction separately and in combination (1:1) of ointment and gel preparations was investigated in vivo, supported by histopathological examinations and biomarker evaluation, as well as molecular association studies for the main components. Topical application of G. glabra ointment and gel, S. japonica ointment and gel, and combination preparations significantly increased the rate of wound healing and reduced oxidative stress in the wound area by reducing MDA and increasing reduced GSH and SOD levels. to the wound and Nolaver ®-treated groups. A molecular docking study showed that the main compounds of G. glabra and S. japonica can effectively bind to the active sites of three proteins involved in wound healing: glycogen synthase kinase 3-β (GSK3-β), matrix metalloproteinases-8 (MMP). -8) and Nitric Oxide Synthase (iNOS). Consequently, G. glabra roots and S. japonica leaves can be a rich source of bioactive metabolites with antioxidant, anti-inflammatory and wound healing properties [8]. Calendula officinalis L. (marigold) flower oil is the main preparation used in cosmetic products and contains terpenoids and terpenes (mainly bisabolol, faradiol, xamazulene, arnidiol and ethers), carotenoids (mainly with rubixanthin and lycopene structures), including flavonoids (mainly quercetin) has a number of bioactive compounds. Isorhamnetin and kaempferol aglycones) and polyunsaturated fatty acids (mainly calendic acid).
Calendula officinalis L. has been used for medicinal purposes since the twelfth century. The plant is reported to exhibit several biological activities such as angiogenic, vascular regeneration, analgesic, antimicrobial, antioxidant and immunomodulatory. In cosmetic products, calendula is used in sensitive skin and soothing products (e.g., after-sun products) among various presentations, including skin, eye, hair, and bath products with recognized safety for use in cosmetics [9-11]. Several Calendula preparations are available for inclusion in topical preparations aimed at healing wounds and soothing inflamed and damaged skin, such as extracts, tinctures, and oils. In the catalog of European cosmetic ingredients, there are 14 INCI designations for Calendula officinalis L. preparations, and within the same designation, it is possible to find botanical preparations with different ingredients depending on the part of the plant and the extraction method. Calendula flower extract is most often used in cosmetic products. Although Calendula officinalis L. is often prescribed for its anti-inflammatory activity, few studies have been conducted on this activity. Interestingly, there are no reports on the activity of calendula extracts in inhibiting the potent pro-inflammatory mediator Nitric Oxide (NO) produced by macrophages in inflammatory-related pathologies. Inflammation is triggered by many pathophysiological conditions in response to microorganisms and tissue damage.
In the initial phases of this process, the first line of defense is provided by macrophages, which, in the presence of a stimulus such as microbial Lipopolysaccharide (LPS), a Toll-like receptor 4 agonist, release several pro-inflammatory mediators, including nitric oxide, cytokines, and prostaglandins. Under normal conditions, the release of these molecules is of great importance, manifesting itself during severe, rapid and only short-term injury until the resolution of the noxious stimuli. However, long-term abnormal production of these proinflammatory mediators can lead to chronic inflammation-related diseases. Therefore, molecules capable of reducing the production of these pro-inflammatory mediators, including NO, offer anti-inflammatory potential [9,11]. Evaluate the effect of Red Clover Extract (RCE) isoflavones on the subjective condition of the skin, appendages, and several mucous membranes in postmenopausal women. Method. Postmenopausal women (n = 109) were randomly assigned to receive either two daily capsules of the active compound (80mg RCE, Group A) or a matching placebo (Group B) for 90 days. After a 7-day washout period, the medication was discontinued and continued for another 90 days. Subjective improvement of the condition of the skin, appendages, and several mucous membranes was assessed for each study group at 90 and 187 days using a Visual Analog Scale (VAS). In addition, libido, fatigue and urinary, sleep and mood complaints were also assessed. Results. After the RCE intervention, women (both groups) reported greater subjective improvement in scalp hair and skin condition, libido, mood, sleep, and fatigue.
Improvements in urinary complaints, nails, body hair, and mucous membranes (mouth, nose, and eyes) did not differ between treatment phases (within and between groups). Overall satisfaction with treatment was reported to be higher after the RCE intervention (both groups) compared to placebo. RCE supplementation has been shown to improve scalp hair and skin condition as well as libido, mood, sleep and fatigue in postmenopausal women [12].
“Glysotrical” gel, prepared in the laboratory of pharmaceutical technology of AMU, was ingested as a research object. The identity of the drug was determined by chromatography on a thin layer, and pH was determined by the potentiometric method. The determination of rutin and glycyrrhizic acid in the studied gel was carried out by the High-Performance Liquid Chromatography (HPLC) method (on the Agilent 1100 American chromatograph at 360 and 260 nm wavelengths). Rheological properties of Glysotrical gel were studied in “Reotest-2”, melting temperature Ubbelode device. Inspections related to microbiological purity were carried out in accordance with the requirements of the XIII State Pharmacopoeia [13-15].
Glysotrical gel is dark brown in color and has a specific tuberculous smell. To determine its identity, 5.0g of the drug is placed in a flask with a volume of 50ml, 20ml of water is added to it, it is kept on a water bath at a temperature of (38 ± 5)°C, with continuous stirring, kept for 15 minutes and then cooled to room temperature. The obtained solution is filtered through a paper filter. The filtrate is evaporated to a dry residue in a porcelain dish, dissolved in 5 ml of 95% ethyl alcohol (or methyl alcohol) heated to 60-70°C and filtered into a 10 ml flask. The volume is brought up to 5 ml by washing the filter paper with a suitable solvent (solution I). 0.025 g of standard rutin is dissolved in 2 ml of boiling methyl alcohol (solution II). 0.02 g of standard glycyrrhizic acid is dissolved in 2 ml of 60% ethyl alcohol (solution III). 0.06 ml of solutions I and II are brewed on the starting line on the KCK No. 2 “Silufol” plate (DS 39560-76) fixed with a silica gel layer measuring 8x12cm. The chromatography plate was air-dried for 15 minutes, then placed in a chromatography chamber containing n-butanol-glacial acetic acid-water (4:1:1). After the mixture of solvents has traveled a distance of 10-15cm, the chromatography plate is removed from the chamber, dried in a drying cabinet at 105°C for 15 minutes. Then the chromatogram is treated with ammonia vapors. In a given solvent system, the rutin contained in the drug and the standard rutin should have the same Rf. Viewed in UV light at a wavelength of 363nm. The yellow-brown spot of rutin with Rf close to 0.7 is noted.
Similarly, 0.06 ml of I and III solutions are poured onto the starting line on the KCK No. 2 “Silufol” plate (DS 39560-76) fixed with a silica gel layer measuring 8x12cm. The chromatography plate was air-dried for 15 minutes, then placed in a chromatography chamber containing n-butanol-glacial acetic acid-water (4:1:1). After the mixture of solvents has traveled a distance of 10-15cm, the chromatography plate is removed from the chamber, dried in a drying cabinet at 105 0C for 15 minutes. Then the chromatogram is treated with a 25% alcoholic solution of phosphorous-tungstic acid. Glycyrrhizic acid and standard glycyrrhizic acid should have the same Rf in the given solvent system. Viewed in UV light at a wavelength of 260nm. The location of the light purple spot belonging to glycyrrhizic acid with Rf close to 0.88 is noted. The rheological properties of “Glysotrical” gel were studied with the help of a rotational viscometer. “Reotest-2” is a two-system device. The rotational viscometer allows determining the rheological properties of liquids in a wide range, which is mainly typical for non-Newtonian liquids. The rheological properties of the gels we prepared with different structuring agents (chitosan, Na-KMS) were studied. In the course of work, preference was given to chitosan, which has a natural and complex structure, is close to the tissue of the human body, does not lose its stability when stored, and has a therapeutic effect as a structuring agent. Cylinder S2 was selected for the study. 30 ml of chitosan- containing gel was added to the measuring tube.
After the connecting end of the cylinder is attached, the measuring tube is also attached to the main unit, a large volume cylinder equipped with water and thermostatic tubes, and a thermometer is attached to the main unit and the device is started. By adjusting the temperature regime (20, 40, 60°C), the rheogram of the gel is established. The dynamic viscosity of the gel should be -984sPs at 10°C, -898sPs at 22°C. The pH of the studied gel should be from 5.5 to 6.0 (potentiometric method, XIII Russian State Pharmacopoeia). The HPLC method was used to determine the amount of rutin and glycyrrhizic acid in “Glysotrical” gel. (Figures 1 & 2) show the chromatograms of the standard rutin and glycyrrhizic acid, and (Figures 3 & 4) show the chromatograms of the sample solution. The amount of rutin in 1 g of the preparation is determined by the following formula:
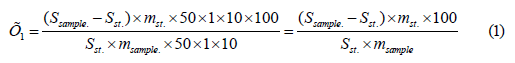
Here, Ssam. is the average of peak areas of rutin calculated in the chromatogram of the sample solution under study.
Sst. - the average of peak areas of rutin calculated in the chromatogram of the Working Standard Sample solution of rutin; rutin.
mst- the weight of the rutin taken for the preparation of the Working Standard Sample of the rutin, in g.
msam. – the sample weight of the drug, in grams.
The amount of rutin in 1 g of the preparation should be from 9.51% to 9.54%. The amount of glycyrrhizic acid in 1 g of the preparation is determined by the following formula:
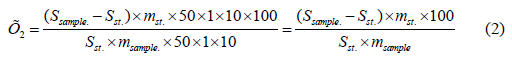
Here, Ssample is the average peak area of glycyrrhizic acid calculated in the chromatogram of the sample solution under study.
Sst. – the average indicator of peak areas of glycyrrhizic acid calculated in the chromatogram of working standard sample solution of glycyrrhizic acid.
acid.- the weight of glycyrrhizic acid taken for the preparation of the Working Standard Sample of glycyrrhizic acid, in grams.
msample – the sample weight of the drug, in grams.
The amount of glycyrrhizic acid in 1 g of the preparation should be from 8.31% to 8.34%. Similar experiments were repeated 6 times to determine the reliability and accuracy of the mentioned method. The obtained results are given in (Tables 1 & 2). As a result, it was found that the amount of rutin in “Glysotrical” gel is 9.52%, and the relative error is 0.19%. The result of the analysis revealed that the amount of glycyrrhizic acid in “Glysotrical” gel is 8.31%, and the relative error is ±0.18%. The HPLC method applied for quantitative determination, unlike other classical methods, is fast, has high accuracy and selectivity. To determine the amount of gel in tubes or vials, 3 tubes (vials) filled with gel are weighed separately with an accuracy of 0.01g. Then the gel mass is emptied, the inside of the tubes is washed with hot water, dried through a filter paper, and empty tubes (vials) are drawn. The amount of gel in each tube (vial) should be 29.5g. The microbiological purity of the preparation is checked based on the requirements of the XIII State Pharmacopoeia. For this purpose, “Glysotrical” gel was dissolved in physiological solution at a ratio of 1:1 and diluted at ratios of 1:10, 1:100, 1:1000. From the rinsed basic substances and each dilution, they were planted on nutrient media - meat-peptone agar, sugar agar, 5% sheep blood agar, Endo, Saburo media. The petri dish with Saburo’s medium was incubated at 28°C for 7 days, and the other dishes were incubated at 37°C for 2 days in a thermostat. Plates incubated at 37°C were stored at room temperature at 20-25°C for 5 days (due to the possibility of pigment formation and fungal flora development) and the results were recorded. It was found that sugar agar contained bacterial colonies (103 in 1g of preparation), as well as yeast and mold fungi (102 in 1g of preparation) in “Glysotrical” gel. This also corresponds to the requirement of XIII SP. The presence of bacteria from the families of Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacteriaceae is not allowed in the preparation.
Table 1: Metrological characteristics of rutin determined by HPLC method in “Glysotrical” gel (msamp. =0.2106; Ssamp. -1614.38; Sst.=986.092).
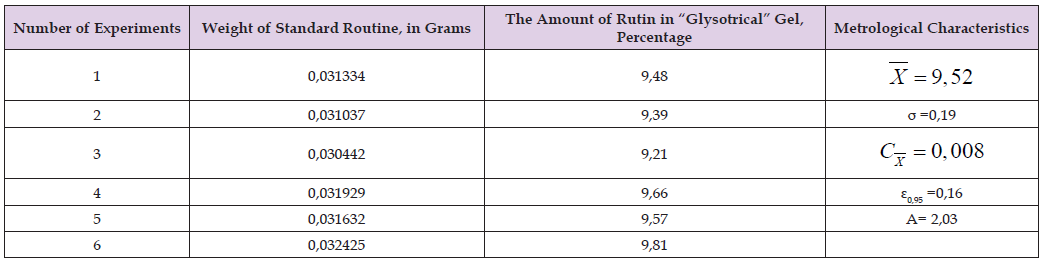
Metrological characteristics of rutin determined by HPLC method in “Glysotrical” gel (msamp. =0.2106; Ssamp. -1614.38; Sst.=986.092).
Table 2: Metrological characteristics of glycyrrhizinic acid determined by YEMX method in “Glysotrical” gel (msamp.=0.2106 g; Ssamp.=1950.76; Sstandart=1065.59).
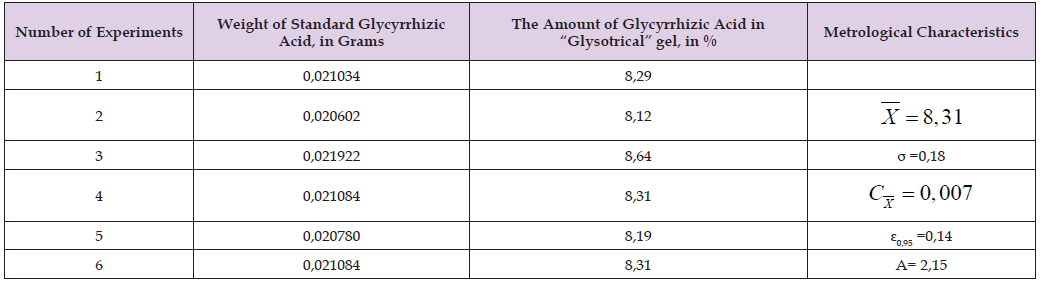
Note: Chromatography conditions: speed of the mobile phase - 1 ml/min; tube temperature-23-27°C; measure- 260 nm wavelength for glycyrrhizic acid.
“Glysotrical” gel was studied by checking the shelf life in 2 series. For this purpose, the gel dosage form from the first series is placed in glass (plastic) vials (6 pieces) at 22°C, and the gel dosage form from the second series is placed in glass vials and stored at 10°C. Organoleptic properties, viscosity, melting temperature, pH, amount of rutin and glycyrrhizic acid, which are the main active ingredients, etc., of “Glysotrical” gel in both conditions at the beginning. parameters are set, the results are recorded. After that, once every 4 months, samples are taken from the gel drug form in the next vials and the parameters indicated at the beginning are checked again. As a result of the operations carried out for 24 months, it was clear that it is possible to ensure the stability of the gel when stored in a dark place at temperature regimes of 10 and 22°C. The studied gel should be released in 15 g and 30 g capacity medical tubes (TC-64-7-678-90) or orange glass vials. The preparation should be stored in a dry place, protected from light, at a temperature of 10-22°C. The storage period is 2 years. During the chemical and thermal burn model created on rabbits, as a result of treatment with “Glysotrical” gel, the healing process of the wound ended faster (Figure 5). It is clear from the diagram that during the treatment of chemical burn in animals with “Glysocal” gel, the rate of healing was 53.73%, while the rate of healing with calendula was 21.92%. During the treatment of thermal burn in animals with “Glysocal” l gel, the healing speed of the burn was 50.2%, while the healing speed with calendula gel was 19.96% (Figures 6 & 7).
During the conducted scientific and research works, it became known that the quality criteria of “Glysotrical” gel, which was prepared for the first time on the basis of raw materials of natural origin of Azerbaijan, met the standard indicators, it is safe, and the shelf life is 2 years. The developed phytogel can be recommended in the treatment and prevention of dermatological diseases of various origins. The gel prepared for the first time was proven to have an effective effect in the treatment of burns of chemical and thermal origin. The developed “Glysotrical” gel is offered to the local pharmaceutical industry due to the selection of a unique composition and high-quality criteria.


