Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Vincent Onuoha*
Received: July 08, 2024; Published: July 19, 2024
*Corresponding author: Vincent Onuoha, Bioluminux Clinical Research, Southampton, GBR, England
DOI: 10.26717/BJSTR.2024.57.009038
The study examines the effectiveness of various vaccination strategies against COVID-19. These strategies can be tailored to specific demographic and regional needs, enhancing vaccination outcomes and contributing to global pandemic control efforts. The study aims to analyze the effectiveness of COVID-19 vaccination strategies, aiming to inform evidence-based public health policies and optimize vaccine distribution and deployment for pandemic control. A comprehensive search strategy was used to identify randomized controlled trials from 2014-2024, excluding low-quality and unrelated studies. Data extraction techniques were used to evaluate bias and methodological quality. The study reveals that COVID-19 vaccination strategies can impact the effectiveness and outcomes of vaccination. Longer intervals and a conditional policy lead to lower cumulative mortality. A booster strategy for the Oxford-AstraZeneca vaccine showed increased efficacy with a 12-week interval. A flexible-dose allocation strategy could prevent 23% to 29% more COVID-19 cases compared to a fixed strategy. Single-dose and two-dose vaccines showed different effectiveness, with single-dose vaccines having 55% and two-dose vaccines having 95%. Delayed second doses could reduce ICU admissions. Age-based allocation prioritization reduced mortality, while degree-based strategies reduced infections, hospitalizations, and deaths more effectively. BNT162b2 and ChAdOx1 nCoV-19 were most effective against asymptomatic infection and hospitalization. The findings provide valuable insights for optimizing COVID-19 vaccination strategies.
Keywords: COVID-19; Comparative Effectiveness; Vaccination Strategies; Immunization Protocols; Immunogenicity
The COVID-19 pandemic has led to the rapid development and deployment of vaccines to combat the virus and reduce morbidity and mortality [1]. Since the first vaccines were introduced in late 2020, vaccination strategies have been refined to maximize public health benefits [2].Vaccination strategies have evolved to reduce infection rates, prevent severe illness, and achieve herd immunity. Key considerations include immunogenicity, efficacy, real-world effectiveness, public health impact, and demographics [3]. Standard dosing intervals, established in clinical trials for vaccines like Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273), have been effective in providing early protection and reducing severe cases and hospitalizations [4]. Shorter intervals allow for quicker vaccination campaigns but may require more frequent boosters [5]. Extended intervals in booster doses can improve logistical efficiency and public compliance. They also enhance immunogenicity, with higher antibody levels and stronger T-cell responses [6]. Mixed vaccine schedules are being explored for flexibility and immune response enhancement, showing comparable or superior immunogenicity to homologous schedules, with some combinations showing enhanced protection against variants [7]. Booster doses are crucial in COVID-19 vaccination strategies to maintain or enhance immunity against new SARS-CoV-2 variants. They rejuvenate the immune system’s memory, increase antibody titers, and restore strong immune responses [8]. Booster strategies can be homologous or heterologous, targeting specific variants [9].
Effective booster strategies reduce the risk of symptomatic infection, severe disease, and hospitalization, ensuring global vaccine equity [10]. Alternative dosing strategies like dose-sparing, extended intervals, and heterologous schedules can improve COVID-19 vaccination efforts, despite supply constraints and logistical challenges [11]. Delaying the second dose of COVID-19 vaccines can enhance immunogenicity and manage supply constraints, but requires careful consideration of demographics, continuous monitoring, and clear public communication [12]. Effective COVID-19 vaccine deployment requires a well-planned prioritization strategy, focusing on vulnerable populations, maintaining healthcare capacity, and reducing transmission. Continuous adaptation and equity-focused approaches maximize vaccine benefits across all population segments [13]. Research on COVID-19 vaccination strategies is crucial for improving public health outcomes and optimizing immunization protocols. Key gaps include long-term effectiveness, effectiveness against emerging variants, real-world effectiveness in diverse populations, mixed vaccine schedules, delayed dosing intervals, pediatric and adolescent vaccination strategies, booster doses, vaccine-induced immunity vs. natural immunity, socioeconomic and behavioral factors, and global equity and accessibility. Addressing these gaps will provide a comprehensive understanding of different vaccination strategies, enabling informed public health decision-making. Understanding strategies helps policymakers make informed decisions to enhance the global response to the pandemic and prepare for future public health challenges.
Research Question
Research Question: Based on the research gap described, here are some potential research questions:
“What is the comparative effectiveness of different COVID-19 vaccination strategies, including variations in dose intervals and the use of mixed vaccine schedules, in terms of immunogenicity, efficacy, and real-world effectiveness across diverse demographic groups and geographic regions?”.
Development of Search Strategy: To develop different search combinations, Boolean operations such as “OR” and “AND” were used using the keywords mentioned. A detailed search strategy was employed in which appropriate keywords and database-specific key/indexing terms were used related to “vaccination” OR “vaccine strategy” AND “comparative effectiveness” OR “pandemic control”. PICO criteria were used to formulate the research question to specify population problem, intervention, comparison (if any), and outcomes that were evaluated and considered in the analysis and synthesis of evidence. PICO criteria were followed.
Population and Problem: Individuals eligible for COVID-19 vaccination, including various demographic groups such as age, comorbidities, and geographical regions.
Intervention: Comparative evaluation of different vaccination strategies, including variations in dose intervals (extended vs. standard intervals) and the use of mixed vaccine schedules (heterologous vs. homologous regimens),booster strategies, alternate doses, single and two-dose vaccinations, and prioritization.
Comparison: Comparison of different vaccination strategies against each other and/or against a standard reference, assessing their relative effectiveness in terms of immunogenicity, vaccine efficacy, and real-world effectiveness.
Outcomes: The article discusses the concept of immunogenicity, its real-world effectiveness, and its demographic impact.
Inclusion and Exclusion Criteria: The studies on ethical implications of AI in patient care decisions, autonomy, data privacy, and informed consent. It includes randomized controlled trials, observational studies, meta-analyses, and real-world evidence studies involving human participants who have received COVID-19 vaccines. The studies should evaluate vaccination strategies, compare effectiveness, and report immunogenicity outcomes, vaccine efficacy, and real-world effectiveness. The studies should be published in English or with available translations and published between 2019 and 2024. Exclusion criteria include studies reporting irrelevant outcomes, nonpeer- reviewed publications, animal studies, non-human subjects, and studies on COVID-19 vaccination strategies, vaccine hesitancy, distribution logistics, lack of comparison groups, and studies not available in English or with accessible translations. The studies should be published before a specified cutoff date to ensure relevance.
Database Search and Selection: A search was conducted on several electronic databases, such as Embase, PubMed, and Cochrane Library. The abstracts and title pages of publications were screened to assess their relevance to the review topics, as well as to determine whether they met the requirements for inclusion or exclusion. Relevant studies were obtained by thoroughly examining the complete text and evaluating their appropriateness for inclusion. Use controlled vocabulary and keywords, and use Boolean operators to construct search queries. Report the findings, and discuss implications for healthcare practice, policy, and future research directions. Data Extraction: A well-structured data extraction form was established for the collection of the appropriate information from the included studies. The data was extracted focusing on the Comparative Effectiveness of Vaccination Strategies against COVID-19
Quality Assessment:
Cochrane Risk of Bias Tool (ROB): The Cochrane Risk of Bias tool (ROB) is a systematic approach used in Cochrane Reviews to assess the likelihood of bias in randomized controlled trials (RCTs). The tool evaluates many elements of trials, such as random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessment, insufficient outcome data, and selective reporting. Bias is categorized into three classifications: low, high, or unclear risk. This categorization method enables a thorough review of the research’s strengths and limitations, as well as an assessment of the degree of trust one has in the results [14].
Analysis and Synthesis of Data: The synthesis of the extracted data was done narratively and results regarding the Comparative Effectiveness of Vaccination Strategies against COVID-19 were organized systematically. Subgroup analyses and sensitivity analyses were performed where applicable.
Interpretation of Results: The results were interpreted based on the strengths and restrictions of the studies included in the systematic review. Practical implications and research gaps in the knowledge are explained along with potential suggestions for future research.
Report Writing: The systematic review manuscript was developed using the guidelines of Figure 1: PRISMA Chart (Preferred Items for Systematic Review and Meta-Analysis).The paper was organized under proper headings such as Introduction, Methodology, Results, Discussion, and Conclusions.
Table 1 explains the reason to Excluded studies. Excluded studies are essential for transparency, identifying relevant literature, assessing eligibility criteria, identifying methodological trends, assessing sensitivity analysis, providing contextual information, and resolving conflicts. They enhance the process by providing insights into the research process, identifying gaps in the literature, and facilitating discussions on study eligibility. Several factors led to the exclusion of the research, including Duplicate Data [15], Inadequate Reporting [16], Lack of Relevance [17,18], and excluded Study Design such as systematic review [19,20]. The systematic review summarized in Table 2 encompasses a variety of studies examining different COVID-19 vaccination strategies, their effectiveness, and their impacts. (Liu, et al. [20]) evaluate the strategies of using different dose intervals for COVID-19 vaccination. They concluded that longer dose intervals were more effective than short dose intervals in terms of reducing mortality attributed to several immunological factors in terms of B-cell maturation and Antibody Production. (Moghadas, et al. [21-22]) to find the effectiveness of the length of time between getting the first and second COVID-19 vaccine shots using a dose strategy for vaccines like Moderna Pfizer-BioNTech. They concluded that increasing the length of time between vaccine doses improves hospitalization, and reduces mortality, however, to reduce infection the efficacy and mechanism of the first dose of vaccine is important such as for AstraZeneca Vaccine8- 12 weeks intervals while for mRNA vaccines like Pfizer-BioNTech and Moderna, where a 12-week interval delay is important to reduce disease severity while 9 weeks interval delay is important in reducing hospitalization and death but not disease severity.
Table 1: Explain the reason to Excluded studies. Excluded studies are essential for transparency, identifying relevant literature, assessing eligibility criteria, identifying methodological trends, assessing sensitivity analysis, providing contextual information, and resolving conflicts. They enhance the process by providing insights into the research process, identifying gaps in the literature, and facilitating discussions on study eligibility. Several factors led to the exclusion of the research, including Duplicate Data [15], Inadequate Reporting [16], Lack of Relevance [17,18], and excluded Study Design such as systematic review [19,20].
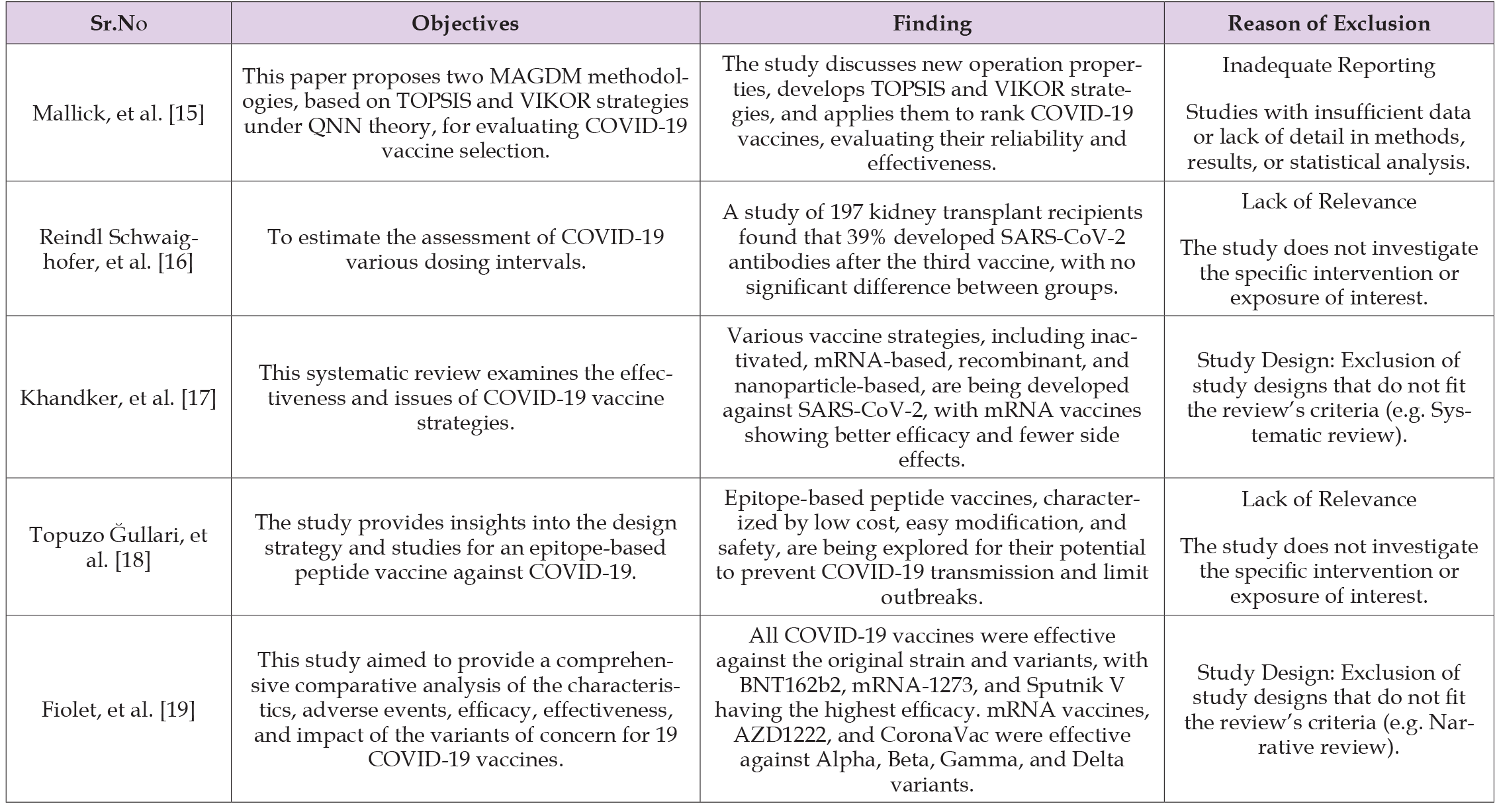
Note: Excluded studies.
(Hung, et al. [23-25]) estimate the effectiveness of adding a booster dose strategy in improving Covid. Antibody levels can rise substantially after a booster dose, providing enhanced protection against the virus. Viral agentsevade immunity from the initial vaccine series, but booster doses enhance the immune response’s potency, enhancing neutralization by increasing Antibody levels. The study also found that vaccine efficacy was higher in participants with a longer first dose-booster dose interval, with binding antibody responses being more than two-fold higher after 12 or more weeks. Such as Oxford– Astra Zenecasingle dose had 76.0% efficacy against symptomatic COVID-19 in the first 90 days, while efficacy increasedfrom 55.1% to 81.3% with a booster dose given after at least 12 weeks. Voysey, et al. [24] found that a booster dose of the SARS-CoV-2 vaccine given 175 days resulted in higher levels of neutralizing antibodies compared to a shorter interval. Tuite, et al. [26] evaluate Alternative dose strategies such as fixed and flexible strategies for COVID-19 vaccine-enhanced immune responses, and better resource utilization. A flexible schedule ensures uniformity in administration, which simplifies logistics and communication with the public. For example, extending the second dose of the AstraZeneca vaccine to 8-12 weeks showed improved efficacy compared to the standard 4-week interval. Paltiel, et al. [27] evaluated the efficacy of several doses of vaccine The study concluded that a single-dose vaccine with 55% effectiveness can stop as many cases as a two-dose vaccine with 95% effectiveness. Anupong, et al. [28,29] evaluate the effectiveness of the Mixed Vaccine strategy in Covid 19. Mixing different types of vaccines can lead to a stronger and more diverse immune response. Anupong, et al. [28] suggested that mixture of heterologous mixture of ChAdOx1 (CV) and CoV-19 (AZ) was more effective than CV and AZ homologous vaccination.
Suphanchaimat, et al. [29] concluded that three-dose schedules (CoronaVac + CoronaVac + ChAdOx1)are more effective than twodose schedules ChAdOx1 + BNT162b2 and CoronaVac + BNT162b2. Abdel-Qader et al., and Soheili et al., evaluate the effectiveness of different types of vaccines against COVID-19 outcomes. BNT162b2 and ChAdOx1 nCoV-19 vaccines demonstrated higher effectiveness against COVID-19 compared to BBIBP-CorV and ChAdOx1 nCoV-19 having high levels of immunology markers. These vaccines are also induced within one month of vaccination, reducing the risk of death. (Soheili, et al. [30]) concluded that the Moderna vaccine had the highest effectiveness, followed by AstraZeneca, Pfizer, and Bharat. mRNA- based COVID-19 vaccines demonstrated the highest total efficacy and effectiveness compared to other vaccines. (Bubar, et al. [31-33]) evaluate the effectiveness of Age-Based and degree-based prioritization for vaccination. Bubar, et al. [31] found that direct vaccination of adults over 60 years of age significantly reduced mortality when transmission was high. Lower transmission vaccination of adults aged 20 to 49 years also reduced mortality and YLL. Buckner, et al. [32] concluded that the priority of younger essential workers leads to control spread and older adults to directly control mortality. A degree- based vaccine strategy refers to prioritizing vaccination efforts based on the social connectivity or “degree” of individuals within a network. Individuals with a higher degree of connectivity (those who interact with many others) are vaccinated first. This strategy leverages the principles of network theory to reduce the spread of infectious diseases more effectively. Chen, et al. [33] concluded that a degree-based vaccine strategy is more important than an age-based strategy to reduce infections, hospitalizations, and deaths, especially in resource-poor countries with lower vaccine availability and slower distribution.
Risk of Bias 2 (ROB 2)
It’s essential to evaluate the quality and potential biases of the included studies to assess the reliability of the evidence. Table 3 explains Risk of Bias of included studies in systematic review. The Risk of Bias 2 (ROB 2) tool is a methodological instrument created by the Cochrane Collaboration to evaluate the potential for bias in randomized controlled trials (RCTs). It assesses the internal validity of research by examining several areas where bias might arise. The instrument evaluates the randomization method, allocation concealment, blinding of participants and staff, blinding of outcome assessment, inadequate outcome data, selective reporting, and overall bias. Research conducted using proper randomization techniques, such as computer-generated random sequences or random number tables, has a reduced likelihood of bias. There is less chance of bias in studies that use strong allocation concealment techniques, such as centralized randomization or opaque, sealed envelopes that are numbered consecutively. Personnel and participants are blinded to the interventions to reduce the possibility of bias. Blinding of outcome assessors reduces the risk of biased outcome assessment. Incomplete outcome data is assessed to determine if there are missing data and how they were handled. Selective reporting ensures that all pre-specified outcomes are reported to avoid selective reporting bias. Overall, the study’s bias is rated as a minimal, moderate concern, or strong based on the evaluations of these areas. The ROB 2 tool provides structured guidance to reviewers to facilitate consistent and transparent assessment of bias in RCTs.
The Systematic review included 15 studies out of which 10 are high-quality studies three studies having some concern while 2 are low-quality studies. Six of the ten included studies are high-quality studies havinga low risk of bias. Random sequence generation is crucial for reducing selection bias. Since only six studies mention this, the remaining four did not report it. Allocation Concealment is adopted in all studies to prevent selection bias. This is a strong point for the validity of these studies. Four studies Utilize double-blind designs where neither participants nor personnel know the group assignments while 2 utilized single blinding where participants do not know the group assignments where neither participants nor personnel know the group assignments. Four of ten studies have Ensured that outcome assessors are unaware of the treatment assignments. Four studies had complete outcome data with no dropouts. While 4 conduct intention-to-treat analyses that include all randomized participants. While 2 studies have some concerns. Eight studies reported all specified outcomes and any deviations from the original protocol in public trial registries.
This summary provides a clear view of the methodological quality across the studies, highlighting strengths in allocation concealment and the absence of selective reporting while identifying areas such as random sequence generation and outcome data completeness that may require closer scrutiny. Two of the ten included studies are low-quality studies with a high risk of bias. Random sequence generation is crucial for reducing selection bias. Since only one study mentioned this, the other one did not report it. Allocation Concealment is adopted in both studies to prevent selection bias. One study Utilize double-blind designs where neither participants nor personnel knew the group assignments while some concerns are present for another study regarding blinding or participants and personnel in group assignments. In one study outcome assessors are aware of the treatment assignments while others have some concerns. Both studies had concerns regarding the drop but they did not report it. Both studies have concerns regarding reporting of all specified outcomes and any deviations from the original protocol in public trial registries. Three of the ten included studies had some concerns regarding the quality of the study. Random sequence generation was mentioned in two studies while the other one did not report it. Allocation Concealment is adopted in all studies to prevent selection bias. One study Utilized double-blind designs where neither participants nor personnel knew the group assignments while no blinding was present for the other two studies regarding group assignments. In one study outcome assessors are aware of the treatment assignments while others have some concerns. All studies having concern regarding drop out they did not report it. All studies reported all specified outcomes and any deviations from the original protocol in public trial registries.
Dose Interval
Modifying dose intervals can potentially offer flexibility in responding to emerging variants, allowing for adjustments in vaccine deployment strategies. Liu, et al. [20] concluded that longer dose intervals were more effective than short dose intervals in terms of reducing mortality attributed to several immunological factors in terms of B-cell maturation and Antibody Production.
Vaccination Strategies with a Delayed Second Dose
Delaying the second dose of a vaccine can increase initial coverage by providing immunity to a larger portion of the population sooner creating herd immunity, especially in areas where vaccine supply is limited. Moghadas et al. [21-22] concluded that increasing the length of time between vaccine doses improves hospitalization, reduce mortality, however to reduce infection the efficacy and mechanism of the first dose of vaccine is important such as for Astra Zeneca Vaccine 8-12 weeks interval while for mRNA vaccines like Pfizer-BioNTech and Moderna, where a 12-week interval delay is important to reduce disease severity while 9 weeks interval delay is important in reducing hospitalization and death but not disease severity.
Booster Dose Strategy
Antibody levels can rise substantially after a booster dose, providing enhanced protection against the virus. Viral agentsevade immunity from the initial vaccine series, but booster doses enhance the immune response’s potency, enhancing neutralization by increasing Antibody levels. The study also found that vaccine efficacy was higher in participants with a longer first dose-booster dose interval, with binding antibody responses being more than two-fold higher after 12 or more weeks. Such as Oxford–AstraZenecasingle dose had 76.0% efficacy against symptomatic COVID-19 in the first 90 days, while efficacy increased from 55.1% to 81.3% with a booster dose given after at least 12 weeks. Voysey, et al. [24] found that a booster dose of the SARS-CoV-2 vaccine given 175 days resulted in higher levels of neutralizing antibodies compared to a shorter interval.
Alternative Dose Strategy
Alternative dosing schedules can be tailored to match logistical capacities and specific public health needs. Tuite, et al. [26] concluded that a flexible schedule ensures uniformity in administration, which simplifies logistics and communication with the public it also allows time to adjust vaccination strategies if new variants emerge that might require different dosing intervals or booster shots. For example, extending the second dose of the AstraZeneca vaccine to 8-12 weeks showed improved efficacy compared to the standard 4-week interval.
Number of Vaccine Doses
The number of vaccine doses in a strategy directly impacts its efficacy, logistical complexity, cost, and public compliance. Single-dose strategies provide rapid coverage but may have lower immunity. Twodose strategies balance efficacy and logistics but may delay full immunity. Three or more doses offer maximum protection but increase logistical challenges and costs. Paltiel, et al. [27] concluded that a single- dose vaccine with 55% effectiveness can stop as many cases as a two-dose vaccine with 95% effectiveness.
Mixed Vaccine Strategy
Mixing different vaccines can sometimes produce a stronger and broader immune response compared to receiving two doses of the same vaccine. Diversifying vaccine sources helps ensure that vaccination efforts can continue even if there are issues with the production or delivery of a particular vaccine. Anupong, et al. [28] suggested that mixture of heterologous mixture of ChAdOx1 (CV) and CoV-19 (AZ) was more effective than CV and AZ homologous vaccination. Suphanchaimat, et al. [29] concluded that three-dose schedules (CoronaVac + CoronaVac + ChAdOx1) are more effective than two-dose schedules ChAdOx1 + BNT162b2 and CoronaVac + BNT162b2.
Types of Vaccine
Different types of vaccines offer various advantages. mRNA vaccines offer high efficacy and rapid adaptability. Viral vector vaccines are easier to distribute and can be effective with a single dose. Protein subunit vaccines have stable storage and low side effect profiles. Inactivated vaccines use an established technology and are easy to store. Abdel-Qader et al. concluded that BNT162b2, ChAdOx1, and nCoV- 19 vaccines demonstrated higher effectiveness against COVID-19 compared to BBIBP-CorV and ChAdOx1 nCoV-19 having high levels of immunology markers. These vaccines are also induced within one month of vaccination, reducing the risk of death. Soheili, et al. [30] concluded that the Moderna vaccine had the highest effectiveness, followed by AstraZeneca, Pfizer, and Bharat. mRNA-based COVID-19 vaccines demonstrated the highest total efficacy and effectiveness compared to other vaccines.
Prioritization
In situations where vaccine supply is limited, prioritization ensures that available doses are used where they can have the most significant impact on public health. Bubar, et al. [31] found that direct vaccination of adults over 60 years of age significantly reduced mortality when transmission was high. Lower transmission vaccination of adults aged 20 to 49 years also reduced mortality and YLL. Buckner, et al. [32] concluded that the priority of younger essential workers leads to control spread and older adults to directly control mortality. A degree-based vaccine strategy refers to prioritizing vaccination efforts based on the social connectivity or “degree” of individuals within a network. Individuals with a higher degree of connectivity (those who interact with many others) are vaccinated first. This strategy leverages the principles of network theory to reduce the spread of infectious diseases more effectively. Chen, et al. [33] concluded that a degree-based vaccine strategy is more important than an age-based strategy to reduce infections, hospitalizations, and deaths, especially in resource-poor countries with lower vaccine availability and slower distribution. The vaccination strategy depends on the specific situation, including vaccine availability, transmission rates, and healthcare capacity. In situations of limited vaccine supply and high transmission, a delayed second dose or prioritization strategy may be most effective in quickly reducing severe cases and transmission. In contrast, when vaccine supply is sufficient, and the focus is on achieving maximum individual protection and long-term immunity, immediate full vaccination might be the preferred approach. Public health authorities must carefully balance these factors to determine the most effective strategy for their specific circumstances.
The study highlights the importance of considering different vaccination strategies for COVID-19. The current study suggested that different dose intervals provide different outcomes. The AstraZeneca Vaccine 8-12 weeks interval while Pfizer-BioNTech and Moderna, where a 12-week interval delay is important to reduce disease severity while 9 weeks interval delay is important in reducing hospitalization and death but not disease severity.
Kayoko Shioda [34] supported the evidence by concluding that the lowest short-term SARS-CoV-2 infection risk, while the late-but-allowable protocol resulted in the lowest risk in the long term, suggesting that delaying the second dose by 1-2 weeks may offer stronger long-term protection. Madhavan [35] also supported the evidence by suggesting that the optimal vaccination strategy depends on the vaccine production rate and first-dose efficacy. Low first-dose efficacy necessitates immediate second-dose vaccination, while high first-dose efficacy requires a time window strategy. The spread rate doesn’t significantly impact the best window thresholds, but significantly impacts total deaths. A current study suggested that a booster dose is more effective in protecting disease in Covid Voysey et al., found that a booster dose of the SARS-CoV-2 vaccine given 175 days resulted in higher levels of neutralizing antibodies compared to a shorter interval. A study in Bogotá [36] showed the same findings that individuals who received a booster dose for COVID-19 detection had a 28% reduction in infection odds. Another study [37] shows similar findings by suggesting that the Pfizer BioNTech booster dose offers significantly increased protection against symptomatic disease in those aged 50 and over, regardless of the initial vaccine.
The current study concluded that Alternative dose strategies such as fixed and flexible strategies for the vaccine enhanced immune responses. Tuite, et al. [26] concluded that extending the second dose of the AstraZeneca vaccine to 8-12 weeks showed improved efficacy compared to the standard 4-week interval. Kho MML, et al. [38] found that repeated booster vaccination is needed to achieve a stronger response to neutralize new virus variants as repeated vaccination increases SARS-CoV-2-specific antibodies. Hill EM, et al. [39] conclude that Vaccines with high protection from the first dose prefer strategies that prioritize one dose, but with increasing vaccine supply, eligible individuals will receive two doses. Optimized dose timing reduces mortality risk, but the logistics of vaccine delivery must be considered. The current study concluded that there is a difference in the effectiveness of vaccination based on number of vaccination doses. Paltiel, et al. [27] suggested that a single-dose vaccine with 55% effectiveness can stop as many cases as a two-dose vaccine with 95% effectiveness. Bhattacharya, et al. [40] supported the evidence by concluding that COVID-19 vaccination provides dose-dependent protection against the development of the disease. It also lowers the risk of hospitalization and ICU admission/death in RT-PCR-positive patients in a dose-dependent manner. A current study concluded thatMixed Vaccines provide more benefits than single vaccines. Anupong. et al. suggested that mixture of heterologous mixture of ChAdOx1 (CV) and CoV-19 (AZ) was more effective than CV and AZ homologous vaccination. Rasheed R, [41] Mixing vaccines from different platforms can enhance IgG and neutralizing antibodies, leading to a stronger cellular immune response. Heterogeneous COVID-19 vaccines have higher neutralizing antibody levels against the virus, prompting both developing and industrialized countries to adopt this mix-and-match strategy to effectively immunize their populations.
The current study concluded different types of vaccines against COVID-19 give different outcomes. The study finding with Abdel Qader, et al. [42] suggested that BNT162b2, ChAdOx1, and nCoV-19 vaccines demonstrated higher effectiveness compared to BBIBP-CorV and ChAdOx1 nCoV-19 having high levels of immunology markers. Xiucui Han, et al. [43] supported the evidence by concluding that different COVID-19 vaccines have unique advantages and disadvantages based on their dosing intervals, efficacy, and side effect profiles. Current study suggested that Age-Based and degree-based prioritization play an important role in vaccination. Bubar et al. Found that direct vaccination of adults over 60 years of age significantly reduced mortality when transmission was high. Chen, et al. [33] concluded that a degree-based vaccine strategy is more important than an age-based strategy to reduce infections, hospitalizations, and deaths, especially in resource-poor countries with lower vaccine availability and slower distribution. Katherine Klise, et al. [44-47] support the evidence by concluding that prioritization strategies can have a large impact on reducing deaths and peak hospitalization, selecting the best strategy depends on community characteristics and the desired objective. Additionally, in some cases, random vaccination performs as well as more targeted prioritization strategies. Understanding these tradeoffs is important when planning vaccine distribution. The findings of comparative effectiveness studies on COVID-19 vaccination strategies have significant implications for public health policy, vaccine deployment strategies, and future research directions. They can help optimize vaccine deployment, enhance effectiveness, address vaccine equity, inform global strategies, support public health messaging, and guide future research. These findings can help optimize vaccine deployment, improve vaccine efficacy, and build public trust in vaccination efforts.
The study has limitations due to design constraints, data heterogeneity, temporal factors, short follow-up duration, confounding factors, limited subgroup analysis, generalizability, publication bias, incomplete data reporting, and potential for conflicting evidence. Addressing these issues requires careful study design, rigorous methodology, transparent reporting, and ongoing evaluation of emerging evidence. The hesitancy, and global cooperation. Policymakers should adopt flexible strategies, and prioritize the comparative effectiveness of vaccination strategies against COVID-19 requires policy implementation, continuous monitoring and research, addressing vaccine equitable distribution, and addressing vaccine hesitancy through transparent communication, education, and community engagement. Prospects include developing variant-specific vaccines, exploring next-generation vaccines, evaluating long-term immunity and booster doses, and integrating vaccination strategies with other public health measures. This dynamic field requires ongoing research and collaboration to control the pandemic.
The research concluded that the effectiveness and results of vaccination can be affected by a variety of vaccination strategies, including different types of vaccines, different dosages, the amount of time that passes between doses, and the order in which vaccinations are administered. Because of the influences of these variables careful consideration is necessary to optimize public health outcomes.


