Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: July 08, 2024; Published: July 18, 2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy
DOI: 10.26717/BJSTR.2024.57.009037
Aspirin is the acetate ester of salicylic acid and acts by binding irreversibly to cyclooxygenase-1 and cyclooxygenases-2. The inhibition of cyclooxygenase-2 mediates antipyretic, analgesic, and anti-inflammatory actions of nonsteroidal anti-inflammatory-drugs and the adverse-effects are caused by inhibition of cyclooxygenase 1 and cyclooxygenase 2. The analgesic-antipyretic dose of aspirin is 325 to 1,000 mg orally 4 times-daily or 6 times-daily. Aspirin is consumed most often at low-doses for cardio-protection and at higher doses as an analgesic, antipyretic, and anti-inflammatory agents. Orally ingested aspirin is absorbed rapidly and the peak concentration is reached in about 1 hour. The efficacy and safely of aspirin, the prophylaxis and the treatment with aspirin and the trials conducted with aspirin have been reviewed. Aspirin is conjugated with glucuronic acid by UDP-glucuronosyltransferase UGT1A6 and is deacetylated into salicylic acid by the cytochrome P-450 CYP2C9. The pharmacokinetics of aspirin have been studied in healthy volunteers who received 100 mg of enteric-coated sustained-release before (pre-prandially) and post (post-prandially) a highcalorie high-fat breakfast and also received 100 mg of aspirin tablets. The elimination half-life of aspirin is 0.43±0.08 hours (pre-prandially), 1.44±0.59 hours (post-prandially), and 4.32±1.04 hours (aspirin tablets). The toxicity induced by aspirin and the treatment of aspirin poisoning have been reviewed. The aim of this study is to review aspirin efficacy and safely, prophylaxis and treatment and trials conducted with aspirin. The metabolism and the pharmacokinetics of aspirin and the toxicity induced by aspirin and the treatment of aspirin poisoning have also been reviewed.
Keywords: Aspirin; Efficacy-Safely; Metabolism; Pharmacokinetics; Prophylaxis; Toxicity; Treatment; Trials
Mechanism of Action of Aspirin
Aspirin is the acetate ester of salicylic acid and acts by binding the prostaglandin synthase enzymes colloquially known as cyclooxygenases. There are two forms of cyclooxygenase: cyclooxygenase-1 and cyclooxygenase-2. The inhibition of cyclooxygenase-2 is thought to mediate, in large part, the antipyretic, analgesic, and anti-inflammatory actions of nonsteroidal anti-inflammatory drugs. Adverse-effects are largely caused by inhibition of cyclooxygenase-1 and cyclooxygenase-2 in tissues in which they fulfil physiological functions such as the gastrointestinal-tract, the kidney, and the cardiovascular system. Aspirin irreversibly binds the cyclooxygenase-1 and cyclooxygenase-2 [1].
Therapeutic Uses of Aspirin
The analgesic-antipyretic dose of aspirin in adults is 325 to 1,000 mg orally 4 times-daily or 6 times-daily. It is rarely used for inflammatory diseases such as arthritis, spondyloarthropathies, and systemic lupus erythematous; nonsteroidal anti-inflammatory drugs with an assumed better gastrointestinal safely profiles are preferred. Aspirin is consumed most often at low-doses for cardio-protection and at higher doses as an analgesic, antipyretic, and anti-inflammatory agent. The anti-inflammatory doses of aspirin, as might be given in rheumatic fever, range from 4 to 8 grams daily in divided doses. The maximum recommended daily dose of aspirin for adults and children, aged 12 years or older, is 4 grams. The rectal administration of aspirin suppositories may be preferred in infants or when the oral route is unavailable. Aspirin suppresses clinical signs and improves tissue inflammation in acute rheumatic fever [1].
Absorption, Distribution, Metabolism, And Elimination of Aspirin
Orally ingested aspirin is absorbed rapidly, partially from the stomach, but mostly from the upper small intestine. The peak plasma concentration is reached in about 1 hour. The rate of absorption is determined by disintegration and dissolution-rates of the tablet administered, by the pH at the mucosal surface, and by the gastric emptying time. Even though aspirin is more ionized as the pH is increased, a rise in pH also increases the solubility of aspirin and thus the dissolution of the tablets and the presence of food delays absorption of aspirin. After absorption, aspirin is distributed throughout most body tissues and intracellular fluids, primarily by pH-dependent processes. Aspirin is transported actively out the cerebrospinal fluid across the choroid plexus. Roughly 90% of aspirin in plasma is bound to proteins, especially albumin. Hypalbuminaemia is associated with a proportionately higher concentration of free aspirin in plasma. Aspirin is rapidly deacetylated to form salicylic acid by spontaneous hydrolysis or by esterases located in the intestinal wall, red blood cells, and liver. The three chief metabolic products are salicyluric acid (the glycine conjugate), the ether or phenolic glucuronide, and the ester or acyl glucuronide. Aspirin and its metabolites are excreted in the urine and the excretion of aspirin is variable and depends on the dose and the urine pH. The elimination half-life of aspirin in plasma is about 20 min [1].
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “aspirin efficacy, safely”, “aspirin prophylaxis”, “aspirin treatment”, “aspirin trials”, “aspirin metabolism”, “aspirin pharmacokinetics”, and “aspirin toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Efficacy and Safely of Aspirin
Aspirin effectively and safely reduced the risk of myocardial infarction in patients without cardiovascular disease, but it did not reduce the risk of all-cause death, cardiovascular death, and stroke, and increased the risk of major bleeding, gastrointestinal bleeding, and haemorrhagic stroke [2]. Among 2,851 patients, 2,289 patients (80.3%) received 100 mg daily of aspirin (low-dose) and 562 patients (19.7%) received ≥ 200 mg daily of aspirin (high-dose). In patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention, discharge on high-dose rather than low-dose of aspirin may increase the rate of major bleeding without providing additional ischemic benefit [3]. Aspirin administered at the daily dose of 100 mg safely prevents thrombotic complications in patients with polycythaemia vera who have no contraindications to such treatment [4]. It was assessed the efficacy and safely of 100 mg daily of aspirin combined with low-molecular-weight heparin in treatment of preeclampsia. The daily dose of 100 mg of aspirin combined with low-molecular-weight heparin treated preeclampsia and may be advantageous to improve blood pressure, 24-hour proteinuria, coagulation function, and it may reduce the adverse-effects in pregnant women without increasing adverse perinatal outcomes [5]. Patients who received 100 mg daily of aspirin had reduced risks of colorectal cancer and gastric cancer and an increased risk of gastrointestinal bleeding [6].
Prophylaxis with Aspirin
Aspirin administered at the daily dose of 100 mg prevented the overall risk of venous thromboembolism without increasing the risk of bleeding [7]. Aspirin administered at the daily dose of 100 mg prevented the venous thromboembolism and major vascular events with improved clinical benefit [8]. Low-dose of aspirin (100 mg daily) for primary prevention of venous thromboembolism must be cautiously prescribed because of the increased risk of major bleeding [9]. There was no statistically significant difference in the frequency or types of traumatic intracranial haemorrhage between patients who received prophylactic aspirin at the daily dose of 100 mg and those who had not. Low-dose of aspirin did not increase surgically relevant parenchymal or meningeal bleeding following moderate and minor head injury in patients aged older than 60 years [10]. Low-dose of aspirin (100 mg daily) administered to patients with limited comorbidities undergoing primary total hip arthroplasty and knee arthroplasty is associated with significant low-rates of bleeding and suture reactions than high-dose aspirin (325 mg daily). Low-dose of aspirin was not inferior to higher dose aspirin for the prevention of venous thromboembolism, wound complications, and infection 90-days postoperatively [11]. Low-dose of aspirin (100 mg daily) was not inferior to high-dose aspirin (325 mg daily) for prophylaxis in patients with venous thromboembolism who underwent total joint arthroplasty. Additionally, patients treated with aspirin for less than 4-weeks may have a higher risk of major bleeding and 90-day mortality compared to patients treated for a longer duration [12].
Comparing low-dose of aspirin (81 mg twice-daily) to high-dose aspirin (325 mg twice-daily) as a venous thromboembolism prophylactic agent, low-dose of aspirin had a lower per-prosthetic joint infection-rate. This may be attributable to a balance of anti-infective properties and antiplatelet effects of aspirin [13]. Aspirin prophylaxis should be considered in patients with hip fracture to mitigate risk of bleeding particularly in patients with low-risk of intermediate venous thromboembolism [14]. Of 36,333 patients undergoing total joint arthroplasty 1,087 patients (3.0%) had a history of venous thromboembolism and did not receive aspirin. The risk of subsequent thromboembolism was significantly higher (P-value = 0.03) in patients with a history of venous thromboembolism (1.4%) compared to patients without venous thromboembolism (0.9%). Aspirin administered at the daily dose of 100 mg is a suitable prophylaxis for the prevention of venous thromboembolism in patients with venous thromboembolism [15].
Treatment with Aspirin
Seven patients with N-ERD received aspirin at the daily dose of 300 mg for 12 years. There was no change in respiratory function parameters following the 12-year aspirin treatment. There was significant improvement in the quality of life and the need for endoscopic sinus surgery due to the recurrence of nasal polyps decreased significantly (P-value < 0.0001). At the 12-year follow-up, all symptoms improved and the improvement in the postnasal drip score was statistically significant (P-value = 0.046). Long-term regular treatment with aspirin at the daily dose of 300 mg in patients with N-ERD improved symptom scores, and alleviated the need for endoscopic sinus surgery due to nasal polyp recurrence [16]. Among 10,018 patients, aged ≥ 65 years, who had no absolute contraindications to aspirin, 6,140 patients (61.3%), with the highest risk of death received aspirin within the first 2 days of hospitalization. The use of aspirin was associated with 22.1% lower odds of 30-day mortality. The use of aspirin in patients with acute myocardial infarction is an excellent opportunity to improve the delivery of care to elderly patients [17]. Children with arthritis received aspirin at the daily dose of 80 to 100 mg/kg and this treatment led to the desired serum salicylate concentration of 200 to 250 µg/ml. Gastric irritation, the most frequent adverse-effect, was significantly reduced by the use of enteric-coated aspirin. At the onset of aspirin therapy in children, there is frequently moderate elevation of SGOT and SGPT liver enzyme levels. With continued treatment, these levels usually fall into normal values [18].
One-hundred-forty-nine sterile women received aspirin at the daily dose of 100 mg and 149 sterile women received placebo. The number of follicles was 19.8+7.2 versus 10.2+5.3 (P-value < 0.05), the number of oocytes retrieved 16.2+6.7 versus 8.6+4.6 (P-value < 0.05), the serum E2 levels were 2,923.8+1,023.4 versus 1,614.3+791.7 pg/ml (P-value < 0.05), the uterine pulsatility index was 1.22+0.34 versus 1.96+0.58 (P-value < 0.05), the ovarian pulsatility index was 1.18+0.31 versus 1.99+0.56 (P-value < 0.05), the pregnancy rate was 45.1% versus 28.2% (P-value < 0.05), and the implantation rate was 17.8% versus 9.2% (P-value < 0.05). Low-dose of aspirin treatment significantly improves ovarian responsiveness, uterine and ovarian blood flow velocity, and pregnancy-rates in women undergoing in-vitro fertilization [19].
Trials Conducted with Aspirin
It was compared aspirin alone to the combination of aspirin plus rivaroxaban for secondary cardiovascular prevention in patients with stable coronary artery disease and/or peripheral artery disease. Five randomized controlled trials involving 33,959 patients were included in the final analysis. These trials showed that rivaroxaban plus aspirin was more effective than the standard therapy of aspirin alone in the prevention of secondary cardiovascular events, major adverse cardiovascular events and/or major adverse limb events, but the combination increased major bleeding. However, the combination treatment was associated with increased of major bleeding [20]. Randomized controlled trials compared the efficacy of aspirin in patients without established atherosclerotic disease. At a mean follow-up of 6.6 years, aspirin was not associated with a lower incidence of all-cause mortality (P-value = 0.300). However, aspirin was associated with an increased incidence of major bleeding (P-value < 0.0001) and intracranial haemorrhage (P-value = 0.001) and with a lower incidence of myocardial infarction (P-value = 0.006). Among patients without established cardiovascular disease, aspirin was not associated with a reduction in the incidence of all-cause mortality; however, it was associated with an increased incidence of major bleeding [21]. It was conducted a randomized, controlled trial to determine if the benefits and risks of aspirin treatment in the primary prevention of cardiovascular disease vary by sex. Aspirin therapy was associated with a significant 14.1% reduction in cardiovascular events (P-value = 0.01) and a 32.0% reduction in myocardial infarction (P-value = 0.001).
There was no significant effect on stroke or cardiovascular mortality. Aspirin treatment increased the risk of bleeding in women (P-value = 0.01) and in men (P-value < 0.001). For women and men, aspirin therapy reduced the risk of a composite of cardiovascular events due to its effect on reducing the risk of ischemic stroke in women and myocardial infarction in men. Aspirin significantly increased the risk of bleeding to a similar degree among women and men [22]. It was performed a multicentre, double-blind trial to investigate the efficacy of 3 aspirin regimens. Patients received low-dose of aspirin (N = 245) and were randomized (1:1:1) to receive 100 mg of aspirin 1, 2, or 3 times-daily for 2 weeks. Patients who received aspirin thrice-daily reported a higher abdominal discomfort score. The currently recommended aspirin regimen of 75 to 100 mg once-daily for cardiovascular prophylaxis appears to be largely inadequate in reducing platelet activation. The antiplatelet response to low-dose of aspirin can be markedly improved by shortening the dosing interval to 12 hours [23]. It was conducted a clinical trial in 11,560 patients with peripheral artery disease. In these patients, low-dose of rivaroxaban plus aspirin is superior to aspirin alone in reducing cardiovascular and limb outcomes in patients undergoing major vascular amputation. This reduction is due to a relative increase in major bleeding. These results support the use of combination low-dose of rivaroxaban plus aspirin in patients with peripheral artery disease [24].
Metabolism of Aspirin
(Palikhe, et al. [25]) observed that aspirin is conjugated with glucuronic acid by UPD-glucuronosyltransferase UGT1A6 and is hydroxylated by cytochrome P-450 CYP2C9. (Bojić, et al. [26]) stated that aspirin is rapidly deacetylated by CYP2C9 into salicylic acid, which forms two hippuric acids: salicyluric acid and gentisic acid (Figures 2 & 3). (Rashid, et al. [27]) studied the metabolism of aspirin in 12 healthy male volunteers who received a single oral dose of 600 mg of aspirin. Aspirin is eliminated mainly by kidney as salicyluric acid (75%), free salicylic acid (10%), salicylic phenol (10%), aspirin glucuronide (5%), and gentisic acid (1%) (Figures 4 & 5). (Navarro, et al. [28]) studied the interindividual variation in aspirin metabolism and observed the effect of sex and ethnicity on the variation in UDP-glucuronosyltransferases. Table 1 shows the urinary excretion of aspirin and its metabolites and their variation according the sex and ethnicity (Table 1). All statistical analysis was performed on log-transformed percent of metabolites recovered (metabolite/total x 100) adjusted for body-mass-index, age, UGT1A6 genotype, sex, ethnicity, and urinary volume and pH. Other ethnicity includes unknown/refused (N = 25), mixed races (N = 42) American Indian (N = 2) and Pacific Islands (N = 3). aP-value < 0.05 male versus female. bP-value < 0.05 versus Caucasian (concentrations do not total 100% due to adjustment). Table 1 presents the mean percent of metabolites eliminated by sex and ethnicity. Statistically significant differences in the ratios of metabolites eliminated were observed between sexes and ethnic groups.
Table 1: Urinary elimination of aspirin and its metabolites according to the sex and ethnicity. Values are the mean±SD, by Navarro, et al. [28].
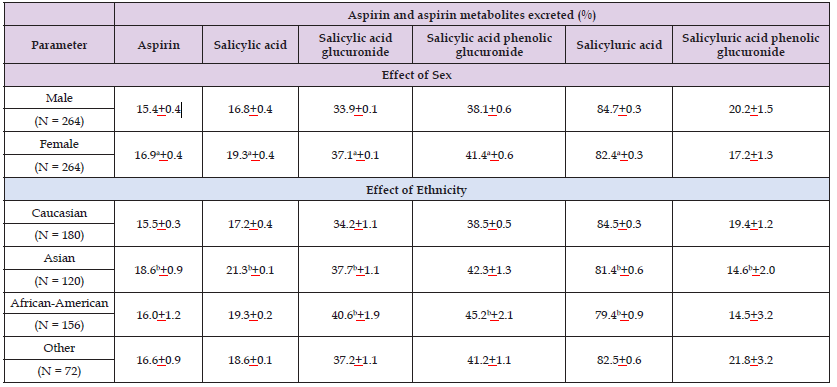
Men excreted more salicyluric acid (3%; P-value < 0.001), and women excreted more aspirin (9%; P-value = 0.03), salicylic acid (11%; P-value = 0.001), and the glucuronides salicylic acid acyl glucuronide and salicylic acid phenolic glucuronide (9 and 10%, respectively; P-value ≤ 0.001 for both). Compared to Caucasians: Asians excreted statistically significantly more aspirin (21%; P-value = 0.002), salicylic acid (23%; P-value < 0.0001) and salicylic acid acyl glucuronide, (11%; P-value = 0.03) and less salicyluric acid (4%; P-value < 0.0001) and salicylic acid phenolic glucuronide (35%; P-vale ≤ 0.002), and African-Americans excreted more salicylic acid acyl glucuronide and salicylic acid phenol glucuronide (16%; P-value < 0.001), and less salicyluric acid (6%; P-value ≤ 0.04). The interaction term for sex*ethnicity was statistically significant for salicyluric acid phenolic glucuronide (P-value = 0.006) and salicyluric acid (P-value = 0.04), with men excreting more of both metabolites than women for all races except African-Americans (data not shown). Excretion of urinary aspirin metabolites did not differ statistically significantly between UGT1A6 genotypes (data not shown). Of the factors evaluated, ethnicity and sex were the greatest, albeit modest, contributors to the variability in aspirin metabolism, and UGT1A6 was not a major factor (Figures 6-9).
Pharmacokinetics of Aspirin
(Cong, et al. [29]) studied the pharmacokinetics of aspirin in 18 healthy volunteers, aged 18 to 45 years, who had a body-mass-index ranging from 19.0 to 26.0 kg/m2. Volunteers were randomly divided into three dosing sequences and received 100 mg of enteric-coated sustained-release aspirin 30 min before (pre-prandially) and 30 min after (post-prandially) a high-calorie high-fat breakfast and received 100 mg of aspirin tablets. Table 2 summarizes the pharmacokinetic parameters of aspirin obtained following the administration of 100 mg of enteric-coated sustained-release aspirin pre-prandially and post-prandially and following the administration of 100 mg of aspirin tablets (Table 2). This table shows that the peak concentration of aspirin is higher in volunteers who received enteric-coated sustained-release aspirin pre-prandially than in volunteers who received enteric-coated sustained-release post-prandially and in volunteers who received aspirin tablets. The peak concentration of aspirin is higher in volunteers who received enteric-coated sustained-release aspirin post-prandially than in volunteers who received aspirin tablets. The time to reach the peak concentration of aspirin is longer in volunteers who received aspirin tablets than in volunteers who received aspirin enteric-coated sustained-release pre-prandially and in volunteers who received aspirin enteric-coated sustained-release post-prandially. The time to reach the peak concentration of aspirin is longer in volunteers who received enteric-coated sustained-release aspirin pre-prandially than in volunteers who enteric-coated sustained-release aspirin post-prandially.
Table 2: Pharmacokinetic parameters of aspirin which have been obtained in 18 healthy volunteers who received 100 mg of enteric-coated sustained-release aspirin pre-prandially or post-prandially or received 100 mg of aspirin tablets. Values are the mean±SD, by Cong, et al. [29].
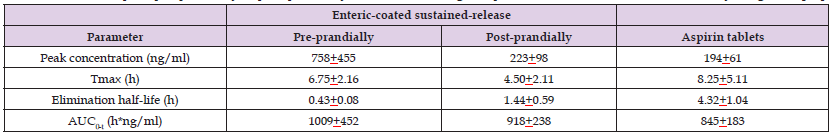
Note: Tmax = time to reach the peak concentration. AUC = area under the concertation-time curve.
The elimination half-life of aspirin is longer in volunteers who received aspirin tablets than in volunteers who received enteric-coated sustained-release aspirin pre-prandially and in volunteers who received enteric-coated sustained-release aspirin post-prandially. The elimination half-life of aspirin is longer in volunteers who received enteric-coated sustained-release aspirin post-prandially than in volunteers who received enteric-coated sustained-release aspirin pre-prandially. The area under the concentration-time curve of aspirin is higher in volunteers who received enteric-coated sustained-release aspirin pre-prandially than in volunteers who received aspirin tablets. The area under the concentration-time curve of aspirin is similar in volunteers who received enteric-coated sustained-release aspirin pre-prandially and in volunteers who received enteric-coated sustained-release aspirin post-prandially. High-calorie high-fat breakfast affects the pharmacokinetic parameters of aspirin in volunteers who received enteric-coated sustained-release aspirin.
Toxicity Induced by Aspirin and the Treatment of Aspirin Poisoning
Aspirin poisoning remains a major clinical hazard, usually resulting from accidental ingestions in preschool children, suicidal overdoses in adults and teenagers, and therapeutically acquired intoxication in all ages. Alkalemia or acidemia, alkaluria or aciduria, hypoglycaemia or hyperglycaemia, and water and electrolyte imbalances may occur. Nausea, vomiting, tinnitus, hyperpnoea, hyperpyrexia, disorientation, coma, and/or convulsions are common [30]. The principal pathophysiologic effect of toxic doses of aspirin are characterized by
1. Stimulation of the respiratory center of the brain, leading to hyperpnoea and respiratory alkalosis;
2. Uncoupling of oxidative phosphorylation, leading to increased oxygen utilization and glucose demand, increased oxygen utilization and glucose demand, increased gluconeogenesis, and increased heat production;
3. Inhibition of Krebs cycle enzymes, leading to decreased glucose availability and increased organic acids;
4. Alterations in lipid metabolism and amino acid metabolism, enhancing metabolic acidosis;
5. Increased fluid and electrolyte losses, leading to dehydration, sodium depletion, potassium depletion, and loss of buffer capacity.
Therapy of aspirin intoxication should be aimed principally at replacement of fluid electrolytes, correction of acidemia, administration of glucose, and enhancement of aspirin elimination [31]. Aspirin overdose causes acid-base disturbances and organ dysfunction. There were 108 patients (79 females, 73.1%) and the median age was 28 years (range: 13 to 93 years). The median dose ingested was 7,750 mg (range, 6,000 to 14,400 mg). Mild toxicity (nausea, vomiting, tinnitus, or hyperventilation) occurred in 22 patients (20.4%) with a median dose of 160 mg/kg. Moderate toxicity (acid-base disturbance, confusion) occurred in 16 patients (14.8%) with a median ingested dose of 297 mg/kg. There were no cases of severe toxicity (coma or seizures) due to aspirin ingestion. The median peak salicylate concentration was 276 mg/l (range, 14 to 814 mg/l). Acute aspirin overdose caused only mild to moderate effects in these patients. Treatment of aspirin poisoning consists in the administration of activated charcoal and sodium bicarbonate [32].
Although the overall mortality in aspirin poisoning is low severe poisoning may cause metabolic acidosis, convulsions, coma, hyperpyrexia, pulmonary oedema, and renal failure. Death can occur in 5% of patients who are severe poisoned and the death is attributable to cardiac arrest or multiple complications after severe brain damage. Critically, in severe aspirin poisoning, delay in diagnosis was associated with a mortality of 15% compared with a much low-rate in those patients in whom early diagnosis and initiation of treatment was made. Elimination of aspirin is increased by alkalinisation of the urine. There is a 10-fold to 20-fold increase in aspirin clearance associated with an increase in urine of pH from 5 to 8. Haemodialysis reduces both the mortality and morbidity in patients with severe aspirin poisoning [33]. Use of low-dose of aspirin is associated with gastroduodenal mucosal damage and increased risk of upper gastrointestinal bleeding.
Many patients on low-dose of aspirin should receive prophylactic treatment because they often present several risk factors that may lead to upper gastrointestinal complications in nonsteroidal anti-inflammatory drug users. The treatment of aspirin poisoning consists in the administration of omeprazole, misoprostol, or high-dose famotidine [34]. Low-dose of aspirin (325 mg or less) is widely used for the management of cardiovascular disease. Although the risk with low-dose of aspirin alone is less than nonsteroidal anti-inflammatory drugs, given widespread use, aspirin related toxicity has become a substantial health care problem due to acute and chronic gas-trointestinal bleeding [35]. Aspirin is the main actor in primary and secondary preventive treatments in cardiovascular diseases. However, it has several adverse-effects including peptic ulcer formation and bleeding. Although lower doses are relatively safe, it should keep in mind that even the lowest dose may cause gastrointestinal bleeding. Gastrointestinal toxicity profile does not differ between conventional and enteric-coated aspirin use [36].
In a patient with impaired renal function, injection of intravenous fluid and urinary alkalization are the mainstays of treatment of aspirin overdose. Repeated doses of charcoal may be a worthwhile intervention when there is no risk of aspiration [37]. Aspirin poisoning is a challenge clinical entity associated with substantial morbidity and mortality. Aspirin is dialyzable by haemodialysis and by emoperfusion and extracorporeal treatment is recommended in patients with severe aspirin poisoning. The intermittent haemodialysis is the preferred modality of extracorporeal treatment. Emoperfusion and continuous renal replacement techniques are acceptable alternatives if haemodialysis is not available [38].
Aspirin is the acetate ester of salicylic acid and acts by binding irreversibly to cyclooxygenase-1 and cyclooxygenase-2. The inhibition of cyclooxygenase-2 mediates the antipyretic, analgesic, and anti-inflammatory actions of nonsteroidal anti-inflammatory drugs and the adverse-effects are caused by the inhibition of cyclooxygenase-1 and cyclooxygenase-2. The analgesic-antipyretic dose of aspirin is 325 to 1,000 mg 4 times-daily or 6 times-daily and aspirin is rarely used to treat anti-inflammatory diseases. Orally ingested aspirin is absorbed rapidly and the peak plasma concentration of aspirin is reached in 1 hour. Aspirin is more ionized as the pH is increased and a rise in pH increases the solubility of aspirin with consequent increase of aspirin absorption. Aspirin is bound to about 90% to plasma protein mainly to albumin and hypalbuminaemia is associated with a higher concentration of free aspirin. The three chief metabolites of aspirin are salicyluric acid (the glycine conjugate), the ether or phenolic glucuronide, and the ester or acyl glucuronide [1]. The efficacy and safely of aspirin have been reviewed. Aspirin effectively and safely reduces the risk of myocardial infarction in patients without cardiovascular disease but does not reduce the risk of all-cause death, cardiovascular death, and stroke and increases the risk of major blending, gastrointestinal bleeding, and haemorrhagic stroke [2], 80.3% of patients received 100 mg daily of aspirin (low-dose) and 19.7% of patients received ≥ 200 mg daily of aspirin (high-dose).
In patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention, discharge on high-dose rather than low-dose of aspirin may increase the rate of major bleeding without providing additional ischemic benefit [3], aspirin administered at the daily dose of 100 mg safely prevents thrombotic complications in patients with polycythaemia vera who have no complications to such treatment [4], the daily dose of 100 mg of aspirin combined with low-molecular-weight heparin treats preeclampsia, improves blood pressure, 24-hours proteinuria, and may reduce adverse-effects in pregnant women without increasing adverse perinatal outcomes [5], and patients who received 100 mg daily of aspirin have reduced risk of colorectal cancer and an increased risk of gastrointestinal blending [6]. These results indicate that aspirin reduces the risk of myocardial infarction but it does not reduce the risk of all-cause of death, cardiovascular death, and stroke, and increases the risk of major blending and haemorrhagic stroke, an aspirin daily dose ≥ 200 mg rather than a daily dose of 100 mg may increase the rate of major blending without providing additional ischemic benefit in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention, the daily dose of 100 mg of aspirin safely prevents thrombotic complications in patients with polycythaemia vera, the daily dose of 100 mg of aspirin combined with low-molecular-weight heparin treats preeclampsia, improves blood pressure, 24-hour proteinuria, coagulation function, and reduces adverse-effects in pregnant women without increasing adverse perinatal outcomes, and the daily dose of 100 mg of aspirin reduces the risk of colorectal cancer and gastric cancer and increases the risk of gastrointestinal bleeding.
The prophylaxis with aspirin has been reviewed. Aspirin administered at the daily dose of 100 mg prevents the risk of venous thromboembolism without increasing the risk of bleeding [7], aspirin administered at the daily dose of 100 mg prevents the venous thromboembolism and major vascular events with improved clinical benefit [8], the daily dose of 100 mg of aspirin prevents the venous thromboembolism and must be cautiously prescribed because may increase the risk of major blending [9], the daily dose of 100 mg of aspirin does not increase surgically relevant parenchymal or meningeal bleeding and minor head injury in old patients with traumatic intracranial haemorrhage [10], the daily dose of 100 mg of aspirin administered to patients with limited comorbidities undergoing total hip arthroplasty and knee arthroplasty is associated with low-rates of bleeding and suture reactions than the daily dose of 325 mg of aspirin. Low-dose of aspirin is not inferior to high-dose of aspirin for the prevention of venous thromboembolism, wound complication, and infection 90-days postoperatively [11], the daily dose of 100 mg of aspirin is not inferior to the daily dose of 325 mg of aspirin for the prophylaxis of patients with venous thromboembolism who underwent total joint arthroplasty. Patients treated with aspirin for less than 4-weeks may have a higher risk of bleeding and 90-day mortality compared to patients treated for a longer duration [12], comparing the dose of 81 mg twice-daily of aspirin to the dose of 325 mg twice-daily of aspirin as a venous thromboembolism prophylactic agent, low-dose of aspirin has lower pre-prostatic joint infection-rate.
This may be due to a balance of anti-infective properties and antiplatelet effect of aspirin [13], aspirin prophylaxis should be considered in patients with hip fracture to mitigate the risk of bleeding particularly in patients with low-risk of intermediated venous thromboembolism [14], of patients undergoing total joint arthroplasty, 3.0% of patients had venous thromboembolism and did not receive aspirin. The risk of subsequent venous thromboembolism is higher (P-value = 0.03) in patients with a history of venous thromboembolism (1.4%) compared to patients without venous thromboembolism (0.9%). Aspirin administered at the daily dose of 100 mg is a suitable prophylaxis for the prevention of venous thromboembolism in patients with venous thromboembolism [15]. These results indicate that the daily dose of 100 mg of aspirin prevents the risk of venous thromboembolism without increasing the risk of bleeding, reduces the rate if recurrence of venous thromboembolism but prevents the major vascular events, prevents the venous thromboembolism but may increase the risk of major bleeding, does not increase surgically parenchymal or meningeal bleeding and minor head injury in old patients, the daily dose of 100 mg of aspirin administered to patients undergoing total hip arthroplasty and knee arthroplasty is associated with lower-rates of bleeding and suture reactions than the daily dose of 325 mg, and the dose of 100 mg daily of aspirin is not inferior to the daily dose of 325 mg of aspirin for the prevention of venous thromboembolism in patients undergoing total joint arthroplasty, patients treated with aspirin for less than 4-weeks have higher risk of bleeding and 90-day mortality than patients treated for longer duration.
Comparing the dose of 81 mg twice-daily of aspirin to the dose of 325 mg twice-daily of aspirin as a venous thromboembolism prophylactic agent, the low-dose of aspirin has lower pre-prostatic joint infection-rate and this may be due to a balance of anti-infective properties and antiplatelet effects of aspirin, aspirin prophylaxis should be considered in patients with hip fracture to mitigate the risk of bleeding in patients with low-risk of venous thromboembolism, the risk of subsequent venous thromboembolism is higher (P-value = 0.03) in patients with a history of venous thromboembolism (1.4%) compared to patients without venous thromboembolism (0.9%). Aspirin prevents venous thromboembolism in patients with venous thromboembolism. The treatment with aspirin has been reviewed. Aspirin was administered at the daily dose of 300 mg for 12 years to patients with N-ERD. There was not change in respiratory function parameters and there was significant improvement in the quality of life and the need for endoscopic sinus surgery due to the recurrence of nasal polyps decreased significantly (P-value < 0.0001). At the 12-year follow-up, all symptoms improved and the improvement in the postnatal drip score was significant (P-value = 0.046). Long-term treatment with aspirin at the daily dose of 300 mg in patients with N-ERD improves symptom score and alleviates the need for endoscopic sinus surgery due to nasal polyp recurrence [16]. Patients, aged ≥ 65 years, with the highest risk of death received aspirin within the first 2 days of hospitalisation and the use of aspirin is associated with 22.1% lower odds of 30-day mortality.
The use of aspirin in patients with acute myocardial infarction is an excellent opportunity to improve the delivery of care in elderly patients [17]. Children with arthritis received aspirin at the daily dose of 80 to 100 mg/kg and this treatment led to the desired salicylate concentration of 200 to 250 µg/ml. Gastric irritation was the most frequent adverse-effect which was reduced by the use of enteric-coated aspirin. At the onset of aspirin therapy SGOT and SGPT liver enzymes increased which fall into the range of mild elevation with the continued treatment [18]. Sterile women received aspirin at the daily dose of 100 mg and other sterile women received placebo. Low-dose of aspirin improves ovarian responsiveness, uterine and ovarian blood flow velocity, and implantation and pregnancy-rates in women undergoing in-vitro fertilization [19]. These results indicate that patients with N-ERD received aspirin at the daily dose of 300 mg for 12 years and there is no change in respiratory function whereas there is improvement in the quality of life and the need for endoscopic sinus surgery due to the recurrence of nasal polyp decreases significantly. Long-term treatment with aspirin in patients with N-ERD improves symptom scores and alleviates the need for endoscopic sinus surgery due to nasal polyp recurrence, the use of aspirin in old patients is associated with 22.1% lower odds of 30-day mortality, in children with arthritis the daily dose of 80 to 100 mg/kg of aspirin produces salicylate concentration of 200 to 250 µg/ml and the gastric irritation is reduced by using enteric-coated aspirin, there is moderate elevation of SGOT and SGPT and with treatment these levels fall into normal values, and a daily dose of 100 mg of aspirin improves ovarian responsiveness, uterine and ovarian blood flow velocity, and implantation and pregnancy-rates in women undergoing in-vitro fertilization.
The trials conducted with aspirin have been reviewed. Five randomized, controlled trials showed that rivaroxaban plus aspirin is more effective than aspirin alone in the prevention of secondary cardiovascular events, major adverse cardiovascular events and/or major adverse limb events but this combination increases major bleeding [20]. Randomized, controlled trials assessed the efficacy of aspirin in patients without established atherosclerotic disease. At a mean follow-up of 6.6 years, aspirin is not associated with a lower incidence of all-cause mortality (P-value = 0.300). However, aspirin is associated with an increased incidence of major bleeding (P-value < 0.0001) and intracranial haemorrhage (P-value = 0.001) and with a lower incidence of myocardial infarction (P-value = 0.006). Among patients without established cardiovascular disease, aspirin is not associated with a reduction in the incidence of all-cause mortality; however, it is associated with an increased incidence of major bleeding [21]. A randomized, controlled trial determined the benefits and risks of aspirin treatment in the prevention of cardiovascular disease by sex. Aspirin therapy is associated with a significant 14.1% reduction in cardiovascular events (P-value = 0.01) and a 32.0% reduction in myocardial infarction (P-value = 0.001). Aspirin treatment increases the risk of bleeding in women (P-value = 0.01) and in men (P-value < 0.001). Aspirin therapy reduces the risk of cardiovascular events in women and in men due to its effect on reducing the risk of ischemic stroke in women and myocardial infarction in men.
Aspirin increases the risk of bleeding to a similar degree in women and in men [22]. A multicentre, double-blind trial investigated the efficacy of 3 aspirin regimens. Patients received low-dose of aspirin (N =245) who were randomized (1:1:1) to receive 100 mg of aspirin 1, 2, or 3 times-daily for 2 weeks. Patients who received aspirin thrice-daily reported a higher abdominal discomfort score. The currently recommended aspirin regimen of 75 to 100 mg once-daily for cardiovascular prophylaxis inadequately reduced platelet activation. The antiplatelet response to low-dose of aspirin can be improved by shortening the dosing interval to 12 hours [23]. A clinical trial was conducted in patients with peripheral artery disease. Low-dose of rivaroxaban plus aspirin is superior to aspirin alone in reducing cardiovascular and limb outcomes in patients undergoing major vascular amputation. This reduction is due to a relative increase in major bleeding. These results support the use of combination low-dose of rivaroxaban plus aspirin in patients with peripheral artery disease [24]. The metabolism of aspirin has been reviewed. Aspirin is conjugated with glucuronic acid by UPD-glucuronosyltransferase UGT1A6 and is hydroxylated by cytochrome P-450 CYP2C9 [25] and aspirin is rapidly deacetylated by CYP2C9 into salicylic acid which forms salicyluric acid and gentisic acid [26]. Following a single oral dose of 600 mg of aspirin, aspirin is eliminated mainly by kidney as salicyluric acid (75%), free salicylic acid (10%), salicylic phenol (10%), aspirin glucuronide (5%), and gentisic acid (1%) [27]. (Navarro et al. [28]) studied the interindividual variation in aspirin metabolism.
Aspirin is metabolized into salicylic acid, salicylic acid glucuronide, salicylic phenolic glucuronide, salicyluric acid, and salicyluric acid phenolic glucuronide and the formation-rate of these metabolites varies with sex and with the ethnicity. (Cong et al. [29]) studied the pharmacokinetics of aspirin in 18 healthy volunteers who received 100 mg of enteric-coated sustained-release aspirin 30 min before (pre-prandially) and 30 min after (post-prandially) a high-calorie high-fat breakfast and received 100 mg of aspirin tablets. The elimination half-life of aspirin is 0.43+0.08 hours (pre-prandially), 1.44+0.59 hours (post-prandially) and 4.32+1.04 hours (aspirin tablets). These results indicate that the elimination-half-life of aspirin is affected by the high-calorie high-fat breakfast. With the exception of the area under the concentration-time curve of aspirin, the peak concentration and the time to reach the peak concentration of aspirin are affected by the high-calorie high-fast breakfast. The toxicity induced by aspirin and the treatment of aspirin poisoning have been reviewed. Aspirin poisoning occurs from accidental ingestion in children, suicidal overdose in adults and teenagers, and therapeutically acquired intoxication in all ages and nausea, vomiting, tinnitus, hyperpnoea, hyperpyrexia, disorientation, coma and/or convulsions are common [30]. The toxicity induced by aspirin causes the alteration of several physiological parameters and the therapy of aspirin intoxication should be aimed principally at replacement of fluid electrolytes, correction of acidemia, administration of glucose, and enhancement of aspirin elimination [31].
Aspirin overdose causes acid-base disturbances and organ dysfunction. The median dose of aspirin ingested was 7,750 mg (range, 6,000 to 14,400 mg) and the mild toxicity is nausea, vomiting, tinnitus, or hyperventilation which occurred following the ingestion of 160 mg/kg of aspirin. The moderate toxicity is acid-base disturbance and confusion and the acute aspirin overdose occurred following the ingestion of 297 mg/kg of aspirin. The treatment of aspirin poisoning consists in the administration of activated charcoal and sodium bicarbonate [32]. Severe poisoning induced by aspirin may cause metabolic acidosis, convulsion, coma, hyperpyrexia, pulmonary oedema, and renal failure and death which occur in 5% of patients who are severe poisoned and death is attributable to cardiac arrest or multiple complications after severe brain damage. The elimination of aspirin is increased with the alkalinisation of the urine. The increase of urine pH from 5 to 8 increases the clearance of aspirin up to 20-fold and the haemodialysis reduces both the mortality and morbidity in patients with severe aspirin poisoning [33]. Low-dose of aspirin is associated with gastroduodenal mucosal damage and increased risk of upper gastrointestinal bleeding. The treatment of aspirin poisoning consists in the administration of omeprazole, misoprostol, or high-dose famotidine [34]. Low-dose of aspirin (325 mg or less) is widely used for the management of cardiovascular disease and the widespread use of aspirin has become a health care problem due to acute and chronic gastrointestinal bleeding [35] and aspirin has several adverse-effects including peptic ulcer formation, bleeding, and gastrointestinal bleeding [36].
In a patient with impaired renal function, injection of intravenous fluid, urinary alkalization, and repeated doses of charcoal are the worthwhile intervention of aspirin overdose [37]. Aspirin poisoning is associated with morbidity and mortality and aspirin overdose is treated by haemodialysis, emoperfusion, and extracorporeal treatment. The intermittent haemodialysis is the preferred modality of extracorporeal treatment and emoperfusion and continuous renal replacement techniques are acceptable alternatives if haemodialysis is not available [38]. In conclusion, aspirin is the acetate ester of salicylic acid and acts by binding the prostaglandin synthase enzymes known as cyclooxygenase. There are two forms of cyclooxygenase: cyclooxygenase -1 and cyclooxygenase-2, cyclooxygenase-2 mediates the antipyretic, analgesic, and anti-inflammatory actions of nonsteroidal anti-inflammatory drugs and the adverse-effects are caused by the inhibition of cyclooxygenase-1 and cyclooxigenase-2, and aspirin irreversibly binds the cyclooxygenase -1 and cyclooxygenase-2. The analgesic-antipyretic dose of aspirin in adults is 325 to 1,000 mg orally 4 times-daily to 6 times-daily and the maximum daily dose of aspirin in adults and in children aged 12 years or older is 4 grams. Orally ingested aspirin is absorbed rapidly, partially from the stomach, but mostly from the upper small intestine and the peak plasma concentration is reached in about 1 hour. The efficacy and safely of aspirin, the prophylaxis and the treatment with aspirin and the trials conducted with aspirin have been reviewed.
Aspirin is conjugated with UDP-glucuronosyltransferase UGT1A6 and is deacetylated by CYP2C9. The pharmacokinetics of aspirin have been studied in healthy volunteers who received 100 mg of aspirin before (pre-prandially) of after (post-prandially) a high-calorie high-fast breakfast and received 100 mg of aspirin tablets. The elimination half-life of aspirin is 0.43+0.08 hours (pre-prandially), 1.44+0.59 hours (post-prandially) and 4.32+1.04 hours (aspirin tablets) indicating that the high-calorie high-fast breakfast affects the elimination half-life of aspirin. In addition, the peak concentration of aspirin and the time to reach the peak concentration of aspirin are affected by the high-calorie high-fast breakfast. The toxicity induced by aspirin and the treatment of aspirin poisoning have been reviewed. The aim of this study is to review the clinical pharmacology of aspirin.
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals.
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.


