Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Fakoya A, Orole R T, Akingbemila A A, Elekan A O, Okoh E F and Olusola A O*
Received: July 04, 2024; Published: July 12, 2024
*Corresponding author: Olusola AO, Department of Biochemistry, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria
DOI: 10.26717/BJSTR.2024.57.009015
Diabetes is a severe metabolic disorder that its management is usually associated with one side effect or the other. Hence, the desire to search for alternative safer drugs by exploring hypoglycemic agents from indigenous plants becomes imperative. This study evaluated the anti-diabetic efficacy of methanol extract of Buchholzia coriacea leaf in streptozotocin (STZ)-treated rats. Thirty male rats (80-145 g) were used in the study and subjected to fasting blood sugar (FBS) test following administration of STZ (45 mg/kg b.w.) for 48 hours. The rats were divided into six groups of five animals each. Group 1 (negative control) was not induced; Group 2 (positive control) were induced but not treated; Group 3 were administered the standard drug, metformin at 50 mg/kg b.w; Group 4 were administered flavonoid fraction (60 mg/kg); Group 5 were administered flavonoid fraction (100 mg/kg); and Group 6 were administered 100 mg/kg of methanol extract of B. coriacea leaf. The animals were sacrificed after 14 days of treatment, and the serum, kidney and liver samples were collected for biochemical analyses. The extract of B. coriacea leaf significantly (p<0.05) reduced alanine aminotransferase, urea and uric acid in the serum of extract treated rats when compared with positive control and metformintreated rats. The activities of aspartate aminotransferase and superoxide dismutase were increased in the liver with a concurrent reduction of liver bilirubin and kidney malondialdehyde levels in the treated rats.
Keywords: Flavonoid Fraction; Biochemical Analyses; Metformin; Serum; Kidney; Liver
Diabetes mellitus is characterized by insufficient blood levels of the hormone insulin. If the blood concentration of insulin is too low, muscle and liver cells will not absorb glucose, resulting to high blood glucose (hyperglycemia). Frequent urination, increased thirst and increased appetite are common symptoms (Mukhtar, et al. [1]). Diabetes can lead to a number of health issues if neglected, including major harm to the heart, blood vessels, eyes, kidneys, and nerves. Acute consequences can result in hyperosmolar hyperglycemia, diabetic ketoacidosis, or even death (Kitabchi, et al. [2]). Heart disease, stroke, chronic renal disease, foot ulcers, nerve damage, eye damage, and cognitive impairment are examples of serious long-term consequences (Saedi, et al. [3]), impaired metabolism of fats and proteins, ketosis and possible diabetic coma (Anderson, et al. [4]). Buchholzia coriacea, commonly known as “Wonderful kola” is a perennial plant which grows as a tree. It belongs to the family Capparaceae and genus Buchholzia (Quattrocchi, et al. [5]).
The seed gave the plant its common name because of its popular usage in traditional medicine (Keay, et al. [6]) such as treatment of diabetes. Previous reports have shown that this plant possesses various medicinal potentials. The analgesic effect of Buchholzia coriacea has been reported (Ezeja, et al. [7]). Also, the hypoglycemic and anti-oxidant effects of the methanol extract of Buchholzia coriacea fruits have been investigated (Okoli, et al. [8]). The anthelmintic potentials of the chloroform and methanol extracts of Buchholzia coriacea seeds have also been reported (Fred-Jaiyesimi, et al. [9]). (Adjanohoun et al. [10]) reported that Buchholzia coriacea is used as a remedy to relieve chest pain in Cameroon. The biochemical pathways leading to these pathologies are not well known but increased oxidative stress is widely accepted as a participant in the development and progression of diabetes and its complications (Oguntibeju, et al. [11]). However, the aim of the study is to investigate the effect of methanol extract of Buchholzia coriacea leaf in streptozotocin-induced diabetic rats and their impacts on both liver function enzymes and antioxidant enzymes.
All chemicals and reagents used for this study were of analytical grade (Figure 1).
Fresh Buchholzia coriacea leaves were obtained from Commander Camp, Odode-Idanre, Ondo State, Nigeria. The plant leaf material was then identified and authenticated by Dr. O. Obembe of the Department of Plant Science and Biotechnology Herbarium (PSBH), Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria with the voucher number of PSB-250 and a sample was later deposited at the Herbarium of the institution for future reference.
Fresh leaves of Buchholzia coriacea were washed with clean water. The washed leaves were air-dried at room temperature for some weeks, pulverized with grinding machine and kept for further analysis.
This was prepared using the method of (Hong et al. 2009). The crude powdered leaf was added to methanol in ratio 1:4 i.e. 500 g of crude powdered leaf to 2000 ml of absolute methanol and was soaked for 72 hours after which it was filtered with cheese cloth and the filtrate was air-dried.
Some portions of the methanol extract were dissolved in a mixture of water and diethyl ether in the ratio 1 to 5 (1:5) inside a separating funnel and was allowed to stand overnight. The aqueous portion was separated and re-partitioned with n-butanol overnight inside a separating funnel. The n-butanol portion was separated the second day and was partitioned with the addition of 1% KOH. The KOH portion was separated and acidified with the addition of dilute HCl partitioned with n-butanol saturated with water. The n-butanol portion (the flavonoid rich extract) was freeze dried at 45 °C.
The presence of flavonoids in the sample was determined by the method described by (Sofowora, et al. [12]). Five millliter (5 ml) of dilute ammonia solution was added to a portion of the aqueous filtrate of the extract followed by the addition of Conc. H2SO4. A yellow coloration observed in the extract indicated the presence of flavonoids
Thirty male albino rats (Rattus norvegicus) with an average weight (80-145) g were obtained from Ajibode Ibadan, Oyo State, Nigeria for this research work. They were housed in a cage under suitable laboratory conditions in the animal house of the Department of Biochemistry, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria. The rats were acclimatized for fourteen days before commencement of any treatment during which they had free access to tap water and food.
An intraperitoneal injection of a low dose of STZ (45 mg/kg body weight) was given to each animal after they had fasted for the entire night.
Two days after STZ injection, blood glucose level was determined using a portable glucometer (Gluco-Plus Inc., Quebec, Canada) in the blood collected from tail vein. Animals with fasting blood sugar (FBS) level > 200 mg/dl were considered diabetic (Ajiboye, et al. [13]).
The rats were administered varying doses of the extract orally for two weeks as shown in Table 1. Metformin (50 mg/kg) served as the reference drug.
The animals were anaesthetized with diethyl ether and were sacrificed by cervical dislocation after two weeks of treatment. Blood samples were collected into EDTA and plain bottles for haematology and serum analysis respectively. The tissues were excised with a clean scalpel, trimmed of fatty tissues, cleansed in normal saline to remove blood stains, weighed and stored frozen at -4 °C for biochemical assays.
Blood samples for serum were allowed to stand at room temperature for 30 minutes to form clot after which it was centrifuged at 3000 x g for 15 minutes. After centrifugation, the clot forms sediments at the bottom of the centrifuge tubes and the supernatant which is the serum was collected using a Pasteur pipette. The serum, thus obtained were appropriately labelled and stored at -5 °C until required for further analysis.
The tissues were homogenized using a homogenizer and were rinsed with normal saline into plain bottles. The tissue homogenates were centrifuged at 3000 rpm for 10 minutes in a cold centrifuge at 4 °C and supernatants were collected and used for biochemical analysis.
Alkaline Phosphatase, Aspartate Aminotransferase and Alanine Aminotransferase were determined using the method of Schmidt and (Schmidt, et al. [14]) as described in commercially available kit produced by Randox. Glutathione level was determined following the method described by (Ellman, et al. [15]). Malondialdehyde level was determined using the method described by (Buege Aust, et al. [16]). Bilirubin determination was carried out following the procedure described by (Walter and Gerard [17]). Urea concentration was carried out following the procedure of (Wheatherburn [18]).
Results are presented as means ± standard error of means of triplicate observations. One-way analysis of variance was conducted followed by post-hoc test using Tukey’s multiple range comparison test obtained from Graphpad Prism 6.0. P-values were set at p<0.05.
Following induction of diabetes by streptozotocin and treatment with 100 mg/kg methanol extract of Buchholzia coriacea leaf, the result of the biochemical assays for the liver function enzymes, urea, uric acid, bilirubin and antioxidant enzymes were presented in Tables 2 & 3 and Table 4 respectively.
The results revealed decrease in glucose level when the rats were administered with 100 mg/kg methanol extract of Buchholzia coriacea leaves (Table 5).
Table 2: The effects of B. coriacea extract on Serum ALT, ALP and Liver AST in STZ-induced diabetic rats.
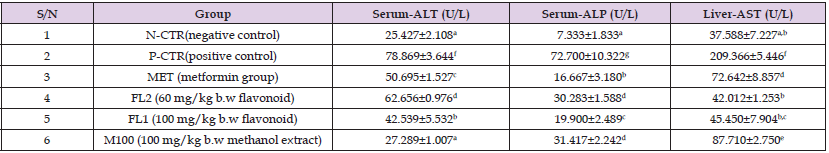
Note: aValues carrying different superscripts are significantly different (p<0.05) from one another, while those bearing the same superscripts do not differ significantly from one another.
Table 3: The effects of B.coriacea extracts on Liver bilirubin, Serum urea and uric acid in STZ-Induced diabetic rats.

Note: aValues carrying different superscripts are significantly different (p< 0.05) from one another, while those bearing the same superscripts do not differ significantly from one another.
Table 4: The effects of B.coriacea extracts on liver SOD activity, GSH and MDA levels in STZ-Induced diabetic rats.
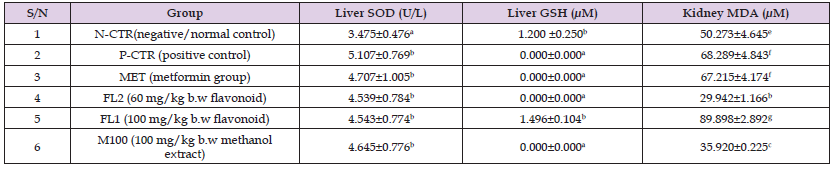
Note: aValues carrying different superscripts are significantly different (p<0.05) from one another, while those bearing the same superscripts do not differ significantly from one another.
Table 5: Average mean weights and glucose levels of rats before and after induction with STZ and the glucose levels after treatment.
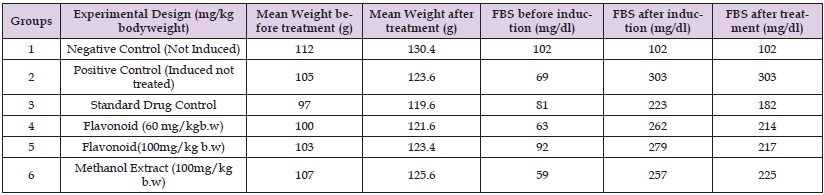
Note: *Results are expressed as mean ± standard deviation p<0.05.
The result outlined in Table 2 shows that serum ALT and ALP as well as liver AST significantly decreases (p<0.05) after treatment with extract of Buchholzia coriacea leaf when compared to negative control group.
The serum urea of rats administered with 100 mg/kg methanol extract was significantly lower when compared to other groups. The serum uric acid was significantly lower than negative control and other treated groups but was not significantly different from that of the positive control group. The bilirubin level was significantly lowered when compared to both positive and negative control but not significantly different from those treated with metformin. As outlined in Table 3.
The activity of SOD in liver in all the treatments were not significantly different from positive control (p<0.05). Contrastingly, the level of GSH was significantly reduced to zero in all treatment except for flavonoid (100 mg/kg b.w) whose level was not significantly different from that of the negative control. Kidney MDA level was significantly decreased (p<0.05) in rats administered with 100 mg of methanol extract of Buchholzia coriacea leaf when compared to both positive and negative controls, metformin group as well as flavonoid group (100 mg/kg b.w) as outlined in Table 4.
Various pharmacological studies on the use of plant for medicinal purposes have been successively studied by several researchers. The use of insulin, glibenclamide, sulphonylurea and other hypoglycemic agents in the treatment of diabetes has been the focused in the modern- day medicine for the treatment of diabetes (Snyder and Berns, et al. [19-21]). Flavonoid-rich extract of Buchholzia coriacea leaf has been shown to lower ALT, ALP, AST, urea and uric acid in the serum of rat in this study. This result appears to support the hepatoprotective effect of this plant against enzymes and urea leakages into the blood stream. This observation was similar to the report of (Ugwu, et al. [22]) who observed decrease in kidney uric acid and creatinine levels of CCl4-induced rats. On the contrary (Lenka, et al. [23]) reported no observable changes in urea and creatinine levels in rats treated with aqueous seed extract of Buchholzia coriacea. Our observation revealed the hepatoprotective efficacy of this plant which may be attributed to the presence of antioxidants phytochemicals such as flavonoids and tannins (Ugwu, et al. [22]).
Contrastingly, the activity of AST was raised in the liver with a concurrent reduction in liver bilirubin level. More so, the increase in the activity of SOD in the liver of rat administered with methanol extract of Buchholzia coriacea leaf is an indication of the response against stress induction, though there was a reduction in the GSH level. Similarly, the observed reduction in the level of MDA in kidney of rat administered with the extract suggests the ability of the plant to offer protection against lipid peroxidation which can also lead to stress in the rat. Ethyl acetate fraction of B. coriacea has been demonstrated to ameliorate oxidative stress in rheumatoid arthritis patient (Alum, et al. [24]). Our results have shown that B. coriacea has the potential to reduced oxidative stress as shown in Table 3. Increased in the activity of superoxide dismutase, reduced glutathione and catalase with corresponding decrease in malondialdehyde level has been previously reported for the lyophilized aqueous seed extract of Buchholzia coriacea (Abraham, et al. [25]).
The study has shown that the extract of B. coriaceae possess antioxidant potentials against any stress induced in diabetes mellitus as evidenced in the level of antioxidant enzymes activities in both the liver and kidney as well as the liver/kidney function enzymes assayed. Hence, B. coriaceae possess hepatoprotective and antixidative potentials against stress induced in diabetes mellitus. These potentials may be associated with the glucose lowering potential of the flavonoid- rich extract of Buchholzia coriacea leaf.


