Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Oumerzouk Jawad* and Cherkaoui Rhazouani Oussama
Received: June 24, 2024; Published: July 03, 2024
*Corresponding author: Oumerzouk Jawad, Professor of Neurology, Street address: University hospitals in Marrakech. Marrakech, Morocco
DOI: 10.26717/BJSTR.2024.57.008989
Background: Ischemic stroke, less common than visual lesions, is a rare but important complication that occurs in 3% to 4% of patients with giant cell arteritis (GCA) and is typically due to stenosis of carotid and/ or vertebral arteries.
Material and Methods: We conducted a multi-center retrospective study in a cohort of 40 patients with GCA, from January 1991 through December 2008, from 2 different neurology departments in Marrakech. 3 patients presented GCA- related cerebrovascular accidents (CVA). All patients with intracranial (IC) involvement received treatment with oral or intravenous glucocorticoids, in combination with an immunosuppressant agent, methotrexate or tocilizumab
Results: In our series, the main clinical presentations in patients GCA with CVA were headache (66.6%), and alteration of visual acuity (100%). Abnormal findings of temporal artery examination were present in 2 patients (66.6%). CVA was more frequent in patients with visual involvement (P = 0.02), especially permanent VL. Furthermore, The patients with intracranial involvement less frequently presented with elevated ESR (33.3%). Posterior circulation arteries were affected in 1 (33.3%) and anterior circulation arteries in 2 (66.6%). Despite treatment, outcomes for patients with IC-GCA were poor. 1 patient (33.3%) with vertebrobasillar ischemic stroke had a rapid progressive disease course and died. The median mRS at followup 6 months after discharge in these patients was 3. Our results support the existence of a clinical subset of GCA patients who are more susceptible to the development of ischemic manifestations.
Discussion: Stroke in GCA patients is directly related to the inflammatory involvement of the Internal Arotid Arteries (ICA), Vertebral Arteries (VA), and more seldom intracranial arteries, and is an uncommon manifestation of GCA, in which the absence of inflammatory syndrome and vision complications seems to be real predictors. Patients with neurologic symptoms and intracranial involvement may have a poor prognosis and fulminant course, even when treated with glucocorticoids and classical immunosuppressive agents, and strokes have been reported to be associated with significant morbidity and mortality as well as residual neurologic deficits in many survivors.
Conclusion: Further studies are needed to draw an increasingly accurate picture of the pathogenesis of GCAs. Such data will be needed to identify new diagnostic biomarkers, improving the diagnostic accuracy of GCAs, and to set up increasingly effective therapies that may avoid severe morbidity and high early mortality in such cases.
Keywords: Headache; Stroke; Visual Loss; Giant Cell; Arteritis; Steroids
Abbreviations: AION: Anterior Ischaemic Optic Neuropathy; AXAV: Axillary Artery Vasculitis; AZA: Azathioprine; CA: Carotid Arteries; CFX: Cyclophosphamide; CIEs: Cranial Ischaemic Events; CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; FP: Fusion Protein; GCs: Glucocorticoids; GCA: Giant Cell Arteritis; GM-SSF: Granulocyte-Macrophage Colony-Stimulating Factor; IC: Intracranial; IHD: Ischaemic Heart Disease; IL: Interleukin; JAK JANUS KINASE; MOAB: Monoclonal Antibody; MMF: Mycophenolate Mofetil; mRS: Modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale; PCA: Posterior Circulation Arteries; PMR: Polymyalgia Rheumatic; PVL: Permanent Visual Loss; R: Receptor; RRA: Recombinant Receptor Antagonist; RTX: Rituximab; S: Stroke; SMOL: Small Molecule; STA: Superficial Temporal Artery; TCZ: Tocilizumab; TVL: Transient Vision Loss; VA: Vertebral Arteries; VC: Vision Complication; ↓: Lower ↑: Higher
Giant cell arteritis (GCA) is a large-vessel vasculitis which affects persons over the age of 50 years, and is known to be common in Nordic populations. Several predisposing genetic factors have been identified, but it has emerged that epigenetic factors are essential in triggering the onset of the disease. It was therefore underlined that GCA should be classified into a cranial form (c-GCA) and an extracranial or large vessel form (LV-GCA). LV-GCA mainly involves the thoracic aorta and its branches and is the main cause of noninfectious aortitis in humans. Despite the systemic nature of involvement, GCA is considered a neuro-ophthalmologic emergency. Intracranial involvement in GCA (IC-GCA) is a rare and highly aggressive disease that is often resistant to steroid monotherapy. Ischemic stroke, less common than visual lesions, is a rare but important complication that occurs in 3% to 4% of patients and is typically due to stenosis of carotid and/or vertebral or basilar arteries. Despite immunosuppressive therapy, patients with intracranial involvement may have a fulminant course with neurological decline and progressing to death. It can be speculated that additional therapies may be available in the future that take advantage of new insights into the pathogenesis of GCA. We report 3 GCA patients with Cerebrovascular Accidents (CVA), and severe prognosis despite immunosuppressants.
We conducted a multi-center retrospective study in a cohort of patients with GCA with intracranial involvement, from January 1991 through December 2008, from 2 different neurology departments at the Mohamed V Military Hospital in Rabat and Avicenne Military Hospital in Marrakech (Morocco). We included 40 patients with the diagnosis of GCA who met the American College of Rheumatology (ACR) classification criteria for GCA. Clinical (average age, sex, GCA symptoms), laboratory and radiological features of all patients with GCA with (n=3) and without (n=37) intracranial involvement included in the study after screening are depicted (Tables 1 & 2). In the present study, we analyzed a large series of patients, in order to 1) assess the frequency, clinical features, clinical characteristics radiological characteristics, pattern of arterial involvement and response to treatment of cerebrovascular ischemic events with outcomes, 2) identify the best predictors for CVA in patients with GCA. Patients with GCA without intracranial involvement (n=37) served as controls. Treatment and outcome information of patients with GCA with intracranial involvement (n=3) included in the study are depicted (Table 3).
Table 3: Treatment and outcome of patients with intracranial involvement.
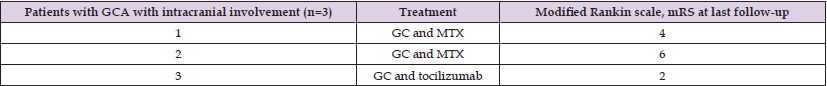
Note: GC, glucocorticoids ; MTX, methotrexate.
The baseline characteristics of the study population are shown in Table 1. We included 3 patients who developed stroke at the same time they presented with GCA symptoms and 37 patients with GCA without intracranial involvement. The median time from symptom onset until diagnosis of GCA was 5 days and did not differ between patients with and without involvement of intracranial arteries. Demographic characteristics did not differ between these two groups. The most frequent symptoms at the time of GCA presentation in patients with cerebrovascular accidents (CVA) were new-onset headache (66.6%), alteration of visual acuity (100%), jaw claudication (33.3%), STA abnormalities (66.6%), constitutional syndrome (33.3%). Polymyalgia rheumatica symptoms were present in 33% of patients. CVA was more frequent in patients with visual involvement (P = 0.02), especially permanent VL. The CVA occurred shortly after the ocular symptoms (median 2.0 days), and the patients with intracranial involvement less frequently presented with elevated ESR (33.3%).
In patients with GCA with intracranial involvement, focal neurological deficits due to intracranial vasculitis at disease onset mainly consisted of motor (33.3%), speech (33.3%) and cerebellar (33.3%) deficits (Table 2). Of 3 patients with GCA with intracranial involvement, neuroimaging showed supratentorial ischemic strokes in 2 (66.6%) patients and infratentorial ischemic stroke in 1 patient (33.3%). Brain magnetic resonance imaging (MRI) performed demonstrated schemic stroke in the territory of the internal carotid artery in 1 patient, middle cerebral artery in one patient, explaining the motor deficit found, and ischemic stroke in the vertebrobasilar territory (cerebellar stroke) in a one patient. Intracranial angiographic imaging modalities used included magnetic resonance angiography and computed tomography angiography (CTA). The most frequently affected arteries were the internal carotid artery (ICA; 33.3%), the vertebral artery (33.3%) and the middle cerebral artery (33.3%) (Table 2). The patient with cerebellar infarction had bilateral vertebral artery stenosis. Abnormal CSF was found in 1 (33.3%) patient including increased total protein.
All patients with intracranial involvement received treatment with oral or intravenous glucocorticoids (1000 g daily for 5 days), in combination with an immunosuppressant agent, methotrexate or tocilizumab and 160 mg daily of Aspirin (Table 3). The mean starting dose of oral prednisone was 52.2 mg daily. Tocilizumab was added to the regimen for the patient with cerebellar infarction (Table 3). illustrates the clinical disease course and treatment strategies in these patients. Response to treatment was characterized by spectacular improvement of symptoms in all patients without intracranial involvement and no relapse during long term follow up (100%). However, despite treatment, outcomes for patients with IC-GCA were poor. In our study, the patient with cerebellar infarction had a rapid progressive disease course characterized by recurrent ischemic events and died within 3 months despite aggressive corticosteroid and supportive therapy. Follow-up 6 months after hospital discharge, showed higher level of disability in GCA patients with intracranial involvement (Table 3).
Giant cell arteritis (GCA) is the most common primary vasculitis affecting the elderly population and constitutes an emergency due to possible devastating neurologic and ophthalmologic complications of the disease. Depending on the arteries primarily involved, the clinical presentation of GCA may vary from constitutional symptoms to amaurosis fugax, jaw, arm or leg claudication, headache, scalp tenderness and eventually stroke. [1-3] Severe cranial ischaemic complications (sCIC), are defined as either severe vision complications [diplopia, transient vision loss and permanent vision defects (permanent vision loss PVL and partial vision field/acuity defect PVF/AD)] or stroke [4]. Visual manifestations of this disease are estimated between 26 and 30%, of whom 14.9% developed permanent visual loss (PVL). PVL caused by anterior ischemic is the best known and most feared complication of GCA. In a retrospective observational study involving a cohort of 123 consecutive GCA patients. 9 (7.3%) experienced ischemic events related to GCA. Of the 9 patients with cerebrovascular events (CVE) caused by GCA, 5 were diagnosed with transient ischemic attacks (TIAs), 2 with ischemic stroke, and 2 were cases involving cranial nerve palsies [5-8]. Amaurosis fugax, stroke and transient ischemic attacks, visual field and acuity changes are among some of the severe complications of the condition. Of these, amaurosis fugax is the most encountered as presenting symptom. However, headache is the most common symptom reported by patients [4,6,9]. New-onset headache in patients aged 50 years and above with elevated erythrocyte sedimentation rate should prompt evaluation for GCA. Constitutional symptoms such as anorexia, fever, weight loss and night sweats are also commonly encountered among patients.
Proximal muscle pain consistent with Polymyalgia rheumatica (PMR) is a characteristic finding making some to believe it a manifestation of the same disease while others construe PMR and GCA as two closely related yet distinct entities. Temporal artery biopsy (TAB) WITH a sensitivity of 77%, is currently the gold standard for GCA diagnosis and demonstrate granulomatous inflammation or mononuclear infiltration and also allows for excluding differentials diagnoses [3,7,10]. Temporal artery ultrasonography is an alternative approach that can be easily performed in most patients. A hypoechoic, thickened temporal artery wall giving a ‘‘halo sign’’has been reported to have sensitivity and specificity of 68% and 81%, respectively in a recent meta-analysis. Colour-Doppler ultrasonography can also be a helpful instrument particularly in the context of a negative Temporal- Artery-Biopsy-Proven (TAB) or missing Superfical Temporal Artery (STA) involvement, revealing parietal thickening of inflammatory vascular origin, carotid and/or vertebral stenoses or occlusions variably associated with hypoechoic mural thickening of the proximal segments, that seems to play an important role in the occurrence of ischemic strokes, since they can be found in many cases with GCA-related ischemic complications [5,11].
The American college of rheumatology 1990 criteria requires 3 out of 5 manifestations; age≥50 years, new-onset localized temporal headache or pain, abnormal temporal artery findings on clinical examination and ESR≥50, positive temporal artery biopsy. The yield is a 93.5% sensitivity and 91.2% specificity. Imaging is an important diagnostic tool in the workup of GCA but must not delay treatment initiation. The 2018 European League Against Rheumatism (EULAR) recommendations are of critical importance for the clinician. With a positive imaging study in a patient with high clinical suspicion of GCA, biopsy can be dispensed with. Ultrasound, positron emission tomography, MRI and computed tomography are important imaging modalities for investigating extra cranial mural inflammation [7,9,11].
The condition is a disruptive immune response to an unknown antigen and has genetic and environmental associations (microbiota dysbiosis or infectious factors). GCA is a T cell-mediated disease with inflammation through all layers of the vessel wall. A crucial role in the pathogenesis of GCA is played by Dendritic Cells (DCs) that reside in the space between the media and adventitia of the arterial wall [3,5,7,8]. Adventitious DCs are activated by unknown molecules of microbial and/or cellular origin. DCs in GCA are typically defective in the expression of the immunosuppressive surface molecule PD-L1. DCs produce IL-12, which promotes the differentiation of Th1 cells, and Il-6 and IL-23, which contribute to the differentiation and stabilization of the phenotype of Th17 cells. T lymphocytes are activated by both DCs and B lymphocytes through the presentation of a putative antigen. The interaction of CD80/CD86 with co-stimulatory receptor CD28, is necessary for T-cell activation. Blocking co-stimulation of T cells by dendritic cells might be a good strategy to inhibit autoreactive T cells, which are probably implicated in the pathogenesis of GCA [10- 13]. Th1 cells produce IFN-γ and GM-CSF, while Th17 cells produce IL-17. These cytokines activate M1 and M1 macrophages, which in turn produce MMP-9, IL1-β, and ROS, contributing to media destruction. Some macrophages, unable to kill the phagocytosed material, transform into giant cells. It should be noted that in GCA, giant cells, which are multinucleated cells after fusion of activated macrophages are the hallmark of this vasculitis. CD4+ and CD8+ Treg cells participate in the inflammatory reaction, being deficient in their immunosuppressive function, as indicated by the stop symbol. CD8+ cytotoxic T cells and neutrophils producing proinflammatory cytokines and NETs play an additional role in the pathogenesis of GCA. At the vascular level, damaged Vascular Smooth Muscle Cells (VSMCs) produce VEGF, PDGF, endothelin-1, and MMP-2, which promote their differentiation into myofibroblasts. These cells cause thickening of the intima and subsequent vascular stenosis.
Many cytokines recognize JAK-associated receptors. Of considerable interest is the role of the Janus kinase/signal transducer and activator of the transcription (JAK/STAT) pathway in the pathogenesis of GCA. Indeed, the JAK/STAT signaling pathway appears to be up-regulated in patients with this vasculitis. Because this transduction pathway leads to nuclear transcription of genes encoding multiple cytokines involved in the inflammatory process. T and B cells Aggregate to form Tertiary Follicular Structures (ATLO), whereas T cells and macrophages are the main components of granulomas [13-15]. It has been shown that vascular stenosis, which is the main complication of GCA, is caused by remodeling of the vessel wall. The tunica media is progressively destroyed, while the intima undergoes thickening due to myofibroblast proliferation and protein deposition in the extracellular matrix, leading to vessel occlusion. A key role of macrophages has been identified in this process. These cells, activated by Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF), in turn activate VSMCs. VSMCs produce Matrix Metalloproteinase (MMP)-2 and MMP-9, which contribute to the destruction of the media and internal elastic lamina. In addition, IFN-γ secreted by Th1 cells activates VSMCs to produce other important factors involved in vascular remodeling. These include Platelet-Derived Growth Factor (PDGF), VEGF, and endothelin-1. PDGF induces the proliferation of VSMCs and their migration into the intimal layer, VEGF promotes angiogenesis, and endothelin-1 promotes the differentiation of VSMCs into myofibroblasts. This complex mechanism eventually leads to intimal hyperplasia and subsequent vessel occlusion.
The role of many of these factors in vascular wall remodeling has been indirectly demonstrated in experimental models using PDGF or endothelin-1 inhibitors that resulted in the blockade of VSMC migration and proliferation [16-18]. The disease process involves maladaptive immunologic response to endothelial injury and tends to affect arteries with elastic tissue within their wall, in which the vasa vasorum represent the door for inflammatory cells. Wilkinson and Russell demonstrated a close relationship between susceptibility to GCA and the amount of elastic tissue present in the arterial wall. Both the innate immune system and the adaptive immune response orchestrate a complex interplay between proinflammatory cytokines, growth factors and various cell types, resulting in systemic inflammation and vascular injury due to structural changes, intimal hyperplasia, thrombus formation, luminal occlusion and ischemic complications. Temporal artery biopsies are graded according to the degree of intimal hyperplasia (grade 1 < 50% luminal occlusion, grade 2 50%-75%, grade 3 > 75%, and grade 4 total occlusion). In each of grades 3 and 4, 75% of patients had neuro-ophthalmic complications compared with 0% for grade 1 and 21% for grade 2 [19-22].
As reported by Weyand et al, this increased susceptibility to ischemic events could be related to the pattern of cytokine expression in the affected temporal arteries, with higher concentrations of interferon ɣ (Th1)- and interlcukin-1ß messenger RNA. Interferon-γ also plays a key role in the pathogenesis of marked intimal hyperplasia, ischemic symptoms and neuro-ophthalmic complications. It should be noted that, unlike Th17 cells, Th1 cells are not sensitive to steroid therapy. This fact confirms an additional unmet need for GCA therapy and justifies the effort to identify new therapeutic agents that can also be effective on this important subgroup of cells [23-26].
Cranial ischemic complications, typically presenting as arteritic optic neuropathy, have been reported in up to one-third of GCA patients. The specific prevalence of CVA in GCA is unknown because in this elderly population, CVA is often attributed to atherosclerosis. The association and temporal relationships of CVA with other ischemic manifestations of GCA, such as PVL and jaw claudication, support the notion that they result from thrombosis or narrowing of the vascular lumen due to arterial wall inflammation. Thus, the prevalence of stroke due to large vessel stenosis in patients affected by GCA is generally low and ranges from 1.5% to 7.2%, as reported in several case series and retrospective studies coming from monocentric or multicentric databases (Table 4). However, only a few studies have assessed the prevalence of GCA among patients affected by ischemic stroke; a recent paper found that 4 out of 2417 patients admitted to a Spanish hospital for stroke had a concomitant GCA. A similar prevalence of CVAs has been reported in studies from different countries, suggesting a negligible role for genetic and environmental factors in the expression of GCA-related brain ischaemic events. Siemonsen et al suggest that cerebrovascular ischemic events in patients with GCA may be more prevalent (20%) than is currently recognized and is frequently asymptomatic. A larger prospective intracranial imaging study of patients with GCA could help clarify the rate of symptomatic and asymptomatic intracranial involvement [27-30].
GCA-related CVAs usually occur within one month of the diagnosis of GCA, and can be prevented by initiating glucocorticoid therapy. Higher prevalence rates were reported when the time frame after the onset of glucocorticoid therapy was extended beyond 4 weeks. However, even in long-term observational studies, the association was strongest in the first month after the diagnosis of GCA, which is consistent with the notion that GCA-related ischaemic events occur most frequently before or shortly after the institution of glucocorticoid therapy. In a large observational cohort study, the risk of an ischemic stroke was nearly 5 times higher in the first month after the diagnosis of CGA compared to control data base patients and only 27% higher in a total follow-up period of several years (median follow-up time 3.9 years). Conn et al suggested that in vasculitis corticosteroids may promote vascular occlusion because platelet thromboxane, relatively unaffected by these agents, could facilitate platelet aggregation and the release of growth factors [31-36].
In a low percentage of cases, stroke may represent the only symptom at onset of GCA. A CVA as the only presenting symptom of GCA is an even more unusual finding (Table 5), with poor prognosis and scarce response to therapy. It is directly related to the inflammatory involvement of the internal arotid arteries (ICA), vertebral arteries (VA), and more seldom intracranial arteries, and is an uncommon manifestation of GCA, particularly feared due to the poor prognosis and severe morbidity. In a study of 98 patients with GCA complicated by CVAs, CVAs represented the initial presentation in 5 out of 68 biopsy- proven cases. Furthermore, in a more recent French multicentric retrospective study, stroke or TIA was found in 18 out of 129 (16%) patients affected by GCA, but only 7 of them suffered from an ischemic event at diagnosis, the other 11 occurring within a year after GCA diagnosis. [28,37-39].
GCA preferentially involves the extracranial branches of the carotid artery as well as the ascending aorta, subclavian and axillary arteries, and vertebral arteries. Involvement of the ocular circulation, supplied by the internal carotid artery, is common, occurring in approximately 50% of patients. The most frequent presentations are arteritic anterior ischemic optic neuropathy (AAION) and central retinal artery occlusion. [40-44]. Ischaemic brain lesions mostly result from vasculitis of the extradural vertebral or carotid arteries. Unlike atherosclerotic disease, the areas of stenoses in GCA typically involve the intracranial distal internal carotid and vertebral arteries, with arteritic involvement abruptly ending a few millimeters distal to the site of dural perforation. The fact that inflammation of GCA diminishes as the vessels perforate the dura may correspond to the thinning of external elastic lamina as the vessel enters the dura with complete loss occurring about 0.5 cm intradurally. The decreased amount of elastin present in intradural vessels may explain the relative sparing of the more distal vessels from the disease. The vertebral artery is a common site of extracranial and intracranial involvement. Overall, 40- 60% of GCA-related strokes involve the vertebrobasilar circulation, compared with 15-20% in the case of strokes caused by atherosclerosis (Figure 1a). The posterior circle is involved in percentages ranging from 46% to 100% of the patients from retrospective studies on three French, two Spanish, one Italian and one Israeli cohorts. The V3 and V4 segment of the vertebral artery were most commonly affected (Figure 1a).
Figure 1:
a) HeatMap with color legend indicating the location of vertebral artery stenosis in GCA patients with intracranial involvement (proximal stenosis) and in patients with atherosclerosis (distal to the origin of posterior inferior cerebellar artery).
b) Heatmaps with color legend illustrating the pattern of internal carotid artery stenosis in GCA patients with intracranial involvement.
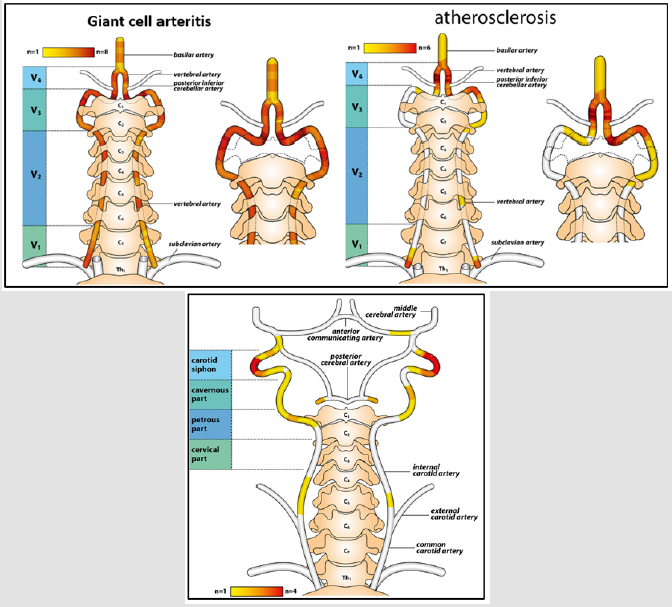
Patients with atherosclerosis were found to have involvement of the V4 segment of the vertebral artery after the PICA origin, whereas in patients with GCA, the V3 segment as well as the V4 segment before the PICA origin were affected. In addition, in GCA stenoses were rather spreading over a long arterial segment in line with the ‘slope sign’ known from axillary artery affection in GCA. This pattern stands in marked contrast to the short-segment stenoses observable in patients with atherosclerosis. Therefore, the differentiation of intracranial GCA from atherosclerosis can be facilitated by the typical pattern of vertebral artery stenosis [42-44].
Strokes most commonly involving the vertebrobasilar system, although carotid arteries may be involved. Internal carotid artery (ICA)-involvement has been reported before as a rare but characteristic pattern in GCA, especially in the petrous and cavernous segments. The first reported case of a patient with GCA, presenting both intracranial/ extradural ICA involvement, was reported by Oerding C and al (2020). Heatmaps of carotid artery involvement showed predominantly bilateral stenosis located within the carotid siphon in GCA patients (Figure 1b). Remarkably, the disease can affect both distal vertebral and internal carotid arteries in a very limited number of cases,with varying clinical presentations [41] In contrast to the ocular circulation, cerebral vessels are spared in this vasculitis, probably because GCA tends to affect arteries with elastic tissue in their wall, and intradural arteries contain little or no elastic tissue in the media and adventitia after dura mater. An additional reason for the rarity of intracranial arteritis in GCA might be the absence of vasa vasorum, through which inflammatory cells enter the vessel wall, from the intracranial arteries. Vasculitis of the intracranial arteries themselves is very rare subset and is associated with severe neurologic deficits and a fatal disease course that usually fails to respond to glucocorticoids [45].
The cause of ischemic events in patients with GCA have been attributed to downstream effects of extracranial vessel stenosis or occlusion of the extradural vertebral and/or carotid arteries that occurs secondary to the inflammatory process or embolization of inflammatory thrombus. The brain infarcts in these patients result from hypoperfusion to the border zones or from artery-to-artery emboli, rather than from arteritis of end vessels. A possible relationship between atherosclerotic disease, common in this age population, and GCA has been proposed as a pathogenic mechanism of the intracranial cerebrovascular disease. Though both processes play a role in the inflammatory process, GCA likely contributes to a higher and earlier mortality by accelerating the progression of vascular disease through impeded collateral flow [43].
In the past two decades, several studies have evaluated the risk factors for vision complications or stroke in GCA (Table 6). Several studies have clearly shown that a significantly lower clinical or laboratory inflammatory activity is associated with a higher risk of developing severe cranial ischaemic events (CIEs), as our study also found. A strong inflammatory response could decrease the risk of ischaemic lesions through the local angiogenic function of proinflammatory cytokines, principally IL-6. A lower tissue expression, as well as a lower circulating level of IL-6, was demonstrated in GCA patients with ischaemic complications compared with those without them [45-47]. Furthermore, a strong correlation between PVL and stroke has been described in some studies and a positive association between traditional atherosclerosis risk factors or established vascular disease and GCA-related ischaemic complications has also been established. Atherosclerosis might contribute to the mechanisms causing ischemic strokes in GCA patients. In our series, the patients with stroke related to GCA had significantly (p=0.02) more atherosclerosis risk factors than patient without CIEs. An emphasized treatment of these risk factors should be considered equally as important as in patients with ischemic strokes of sole atherosclerotic origin [46-48]. Recently, male gender was implicated as a risk factor for vision complications by Ji et al. In our study, male gender did not emerge as an independent predictor of CVA, and Lopez-Diaz et al. reported no significant association between patient age and the frequency of stroke [33,35,49]
As our study found, visual loss may also represents a main contributor of CIEs in several cases. An improved understanding of the risk factors for ischaemic complications could further help to decrease their incidence and to improve patients’ long-term prognosis. Our results support the existence of a clinical subset of GCA patients who are more susceptible to the development of ischemic manifestations. In summary, the predictors of stroke in our study were permanent VL, atherosclerosis risk factors, and absence of inflammatory syndrome [17,22,43,44].
Differential diagnosis between stroke related to-GCA and thromboembolic occlusion is challenging, particularly in the elderly. The characteristic radiographic pattern appears to correlate with the underlying pathologic process. MRI in GCA typically demonstrate increased vessel wall thickness, edema, and increased mural enhancement on post-contrast T1-weighted images and brain ischaemic lesions, whereas magnetic resonance angiography or conventional angiography shows stenoses or occlusions of large intracranial vessels in these patients. Stenosis is classically located at the point of dural entry, but rarely extends intracranially, likely due to the density of intimal tissue in the extracranial portions of these vessels and the relative paucity intracranially. This degree of inflammatory vascular stenosis influences the amount and severity of ischemic strokes by hemodynamic mechanisms. The computed tomography angiogram and MRA may also show the extent of the disease, but are not helpful in the diagnosis of GC [42-44]. Furthermore, there is increasing interest in using Magnetic Resonance Imaging (MRI) of the temporal arteries as an alternative to TAB. A previous series from our centre found that 3T MRI of the scalp arteries had a sensitivity of 94% and specificity of 78% when compared with TAB, with a high negative predictive value of 98% [42-44].
No further relapses and an overall good outcome were noted in all 6 patients from the case series by Zenone et al. However, long-term complications are frequent in patients with neurologic symptoms and intracranial involvement, and may lead to severe morbidity and mortality. The outcome could be poor, with a significant reduction of both survival and remission-free survival, despite high dosage of steroids and immunosuppressants, such as Cyclophosphamide (CFX), Methotrexate (MTX), Rituximab (RTX) and, more recently, Tocilizumab (TCZ). A subset of patients develops a more malignant course with recurrent ischemic strokes from involvement of the distal vertebral or internal carotid arteries. Further CVAs were assessed in 28% of patients from the French multicentric study by de Boysson, despite concomitant immunosuppression. De Boysson, et al. in their retrospective study of 40 patients affected by GCA-associated stroke, reported a mortality rate of 28%, often (63%) within the first 5 days, and a disability rate of 52% among the survivors, with frequent relapses. An even worse outcome is reported among the few cases of intracranial GCA reported in the literature (7 out of 9 patients deceased) and a 100% lethality was observed by Samson et al.in those patients in whom stroke was the presenting symptom, despite high dosage of steroids [41-44].
GCA is an emergency as irreversible neurological and ophthalmological complications could lead to increased morbi-mortality. Guidelines for GCA treatment indicate glucocorticoids, oral or intravenous, depending on severity of symptoms, and aspirin. Patients presenting with GCA, but without cranial ischemic complications, typically respond to steroid monotherapy. Current treatment strategies appear to be of limited efficacy for IC-GCA.
Should be started as soon as possible for initial induction of remission. Oral route requires prednisone at 40 to 60 mg daily (at least 0.75 mg/kg) while IV route requires 1g daily for 3 days followed by oral route. Therapy should not be delayed pending temporal artery biopsy. Early high-dose steroid treatment is important to prevent further visual loss and for rapid control of symptoms, but meaningful recovery from existing visual loss is poor. Gradual steroid dose reduction can be considered in the absence of clinical symptoms and once laboratory inflammation markers have normalized. There is no definite duration of treatment but a minimum of 2 years is a safe duration to prevent relapses. Most patients are able to discontinue steroids after 1 to 2 years of treatment. However, some patients are experiencing a chronic relapsing course [7,17,23,32,40].
Patients affected by GCA are older and often have several comorbidities and long-term corticosteroid therapy is associated with several adverse side effects. Steroid-sparing agents are interesting options. Adding methotrexate, 10 mg weekly, is effective in controlling disease activity, with lower frequency of relapse and lower cumulative dose of steroid. A trial of azathioprine as a steroid-sparing agent in GCA and PMR reported a statistically significant difference between steroid use in the azathioprine group and the control group, but only after a year. Although there are no published studies of leflunomide for treatment of GCA, it has shown promise in a small number of patients with corticosteroid-resistant disease. Despite the scarcity of data about this condition, it should be stressed that classical immunosuppressants are often unable to prevent death and disability. B-cell depletion (rituximab) and cyclophosphamide have been also used as a steroid-sparing strategy [44,46].
New knowledge, albeit partial, has led to the approval of innovative targeted therapy, such as, in particular, the use of the anti-IL6R monoclonal biologic agent tocilizumab (TCZ). Anti-IL-17 antibodies are at an advanced stage of study, and great expectations are placed on JAK inhibitors (figure). To date, TCZ, an interleukin-6 receptor antagonist, is the only biologic agent approved by regulatory agencies for the treatment of GCA due to the results obtained in the Actemra Giant Cell Arteritis Study (GIACTA) and may be administered within the very first days after diagnosis as a first line therapy. TCZ’s efficacy in “classical” GCA has also been proven in several studies after conventional immunosuppressants. However in some cases TCZ was unable to control disease activity, which led to further relapses, severe disability and death. The death of the third GCA patient with vertebrobasilar stroke in our series, confirmed the ineffectiveness of TCZ in some cases of GCA, burdened by frequent relapses and rapid progressive course [44,46] Of particular interest are therapies aimed at inhibiting vascular remodeling. Since GM-CSF appears to be crucial in the destruction of media by macrophages and is involved in the genesis of intimal hyperplasia and neovascularization, its blockade with the specific monoclonal antibody mavrilimumab seems to be an attractive therapeutic option.
Instead, studies are underway on bosentan, an endothelin-1 receptor antagonist. This substance produced by vascular endothelial cells is a potent vasoconstrictor and is involved in the vascular remodeling of GCA [45,47] (Figure 2 & Table 7). Show the the targeted drugs currently approved or under study that inhibit the various factors involved in the pathogenesis of GCA.
Low-dose aspirin was shown to decrease rate of visual loss and cerebrovascular accidents in GCA. Aspirin was shown to suppress proinflammatory cytokines in vascular lesions in GCA. Two recent retrospective studies found that anti-platelet/anti-coagulation therapy reduced the risk of CIEs [8,11,26,37,50].
Endovascular dilatation of stenotic arteries has been successfully proposed in patients unresponsive to high dosages of steroids, but it is presumably less effective in reducing inflammation, potentially leading to further occlusions [9,25,26] Performance of combined pharmaceutical and surgical treatment with an extra-intracranial bypass is an invasive but beneficial option for a well selected group of patients that face progressive hemodynamic impairment and ischemic strokes [2,6,11,23,33] In refractory GCA to usual therapies, whether other aggressive approaches, such as extracranialto-intracranial bypass using arterial grafts, endovascular stenting of arteritic vessels, or autologous hemopoietic stem cell transplant, should be considered in these patients requires further study [43,44].
Cerebrovascular accidents secondary to GCA are an uncommon, difficult-to-treat and are usually associated with significant morbidity and mortality as well as residual neurologic deficits in many survivors. An improved understanding of the risk factors for ischaemic complications and early diagnosis of this disease is beneficial for the patient. Further studies are needed to draw an increasingly accurate picture of the pathogenesis of GCAs. Such data will be needed to identify new diagnostic biomarkers, improving the diagnostic accuracy of GCAs, and to set up increasingly effective therapies that may avoid severe morbidity and high early mortality in severe cases of GCA-related stroke.
Interpretation and comparison of response to treatment is limited by the retrospective design of the study and the comparably small simple size.
This research received no external funding.
The authors declare no conflicts of interest.


