Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Emeka Godwin Anaduakaa, Eleazar Chukwuemeka Anoruea*, Merit Amarachukwu Ezea, Kingsely Chijioke Anekea, Placida Nwabuogo Uliaha, Precious Chinyere Ubakaa and Izuchukwu Marcel Asogwaa
Received: May 22, 2024; Published: June 14, 2024
*Corresponding author: Eleazar Chukwuemeka Anorue, Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, 410001, Nsukka, Enugu State, Nigeria
DOI: 10.26717/BJSTR.2024.57.008941
Background: Anchomanes difformis (AD) of the family Araceae has strong ethnopharmacological relevance
and has been used traditionally against varying pathological conditions, including diabetes, asthma, pain,
wounds, microbial infections, gastrointestinal related problems and kidney pain. However, whereas some of
these folkloric uses and claims have been proven scientifically, others are mere indigenous claims.
Aim: Therefore, this study was carried out to scientifically investigate the therapeutic potentials of Anchomanes
difformis in ameliorating gentamicin induced nephrotoxicity in experimental rats.
Method: The study was divided into four groups consisting of: group 1 (normal control) received 5 ml/kg/day
normal saline; group 2 (positive control) received 5 ml/kg/day normal saline and 100 mg/kg/day gentamicin;
group 3 received 150 mg/kg/day Anchomanes difformis and 100 mg/kg/day gentamicin; and group 4 received
300 mg/kg/day Anchomanes difformis and 100 mg/kg/day gentamicin. Biochemical parameters such as
creatinine, urea, electrolyte and antioxidants makers were investigated together with histopathological
studies.
Result: The result of the study showed that after 14 days of treatment, methanol leaf extract of Anchomanes
difformis was able to ameliorate nephrotoxicity induced by gentamicin. However, there was no complete
reversal of histological damages caused by the gentamicin.
Conclusion: Therefore, methanol leaf extract of Anchomanes difformis could be harnessed for amelioration of
nephrotoxicity induced by gentamicin.
Abbreviations: AD: Anchomanes difformis; HPLC: High-Performance Liquid Chromatography; UPLC-MS: Ultra performance Liquid Chromatography Mass Spectrometry; SOD: Superoxide Dismutase; CI: Cellular Infiltrates
Gentamicin is widely used in the treatment of severe gram-negative bacterial infections (Udupa, et al. [1]). Unfortunately, the clinical use of gentamicin is limited by its potential ototoxicity and nephrotoxicity (Priyamvada, et al. [2]). This is usually characterized by abnormalities in the architecture of the renal tissues, such as glomerular hypertrophy, expansion of the mesangial matrix, thickening of the tubular, and glomerular basement membrane (Ali, et al. [3]). Abnormal values of creatinine, urea, albumin and other proteins in the urine and serum are also typical observations (Udupa, et al. [1]). The mechanism behind gentamicin induced kidney nephrotoxicity is not yet clearly understood. However, because the drug is highly charged and water soluble at physiologic pH, it is unable to diffuse through biologic membrane, hence, selective accumulation of the drug occurs at the renal cortex eventually leading to nephrotoxicity (Zhou, et al. [4]). Temporal variations in the renal toxicity of gentamicin have been reported for experimental animals as well as for humans (Gautier, et al. [5]). In fact, maximal renal toxicity of gentamicin was observed when the drug was given during the rest period, while a lower toxicity was observed when the drug was injected during the activity period (Gautier, et al. [5]). In recent years, gentamicin nephrotoxicity is significantly reduced by shifting to once daily dosage as well as by eliminating known risk factors (Balakumar, et al. [6]).
Also, active hydration with saline and simultaneous administration of mannitol before, during and after gentamicin treatment is a well-known strategy for reducing gentamicin induced nephrotoxicity and associated side effects (Hansen, et al. [7]). However, there is a paradigm shift towards the use of medicinal plants in the management of pathological conditions associated with gentamicin induced nephrotoxicity due to its cost effectiveness, with little or no adverse effects when appropriately used (Balakumar, et al. [6]). Anchomanes difformis (AD) of the family Araceae has a strong ethnopharmacological relevance and has been used traditionally against varying pathological conditions, including diabetes, kidney pains, asthma, pain and wounds, microbial infections, and gastrointestinal related problems (Alabi, et al. [8]). Whereas some of these folkloric uses and claims have been proven scientifically, others are mere indigenous claims (Alabi, et al. [8]). For instance, Agyare and colleagues have reported anti-inflammatory ability of leaves and rhizome extracts of AD on histamine and serotonin; mediators of acute inflammation (Agyare, et al. [9]). They found out that the leaves and rhizome extracts of AD has more anti-inflammatory ability when compared with aspirin as reference drug (Agyare, et al. [9]). Egwurugwu and colleagues have reported the effect of AD rhizome against uterine fibroid (Egwurugwu, et al. [10]). There studies showed that it was able to modulate female sex hormones implicated in uterine fibroid (Egwurugwu, et al. [10]).
Studies by (Adeyemi, et al. [11]) have shown that ethanolic extract of AD root has hypoglycemic effect in alloxan-induced diabetic Wistar rats. Brooks and Oguntibeju (2020) carried out studies on the antioxidant capacities and phytochemical analysis of six different extracts of AD. There studies showed that AD is a natural source of antioxidant with aqueous leaves extract exhibiting the highest antioxidant ability. Phytochemical characterization of the aqueous leave extract using ultra performance liquid chromatography mass spectrometry (UPLC-MS) and high-performance liquid chromatography (HPLC) showed the presence of bioactive compounds including phloridzin, quercetin, rutin, and kaempferol which all have therapeutic effect against inflammation, diabetes, and apoptosis (Alabi, et al. [8]). Presently, no study has investigated the possible effects of AD leaves extract on kidney functional parameters as well as histological damage in kidney nephrotoxicity. This paucity in the literature was a major drive that led to this study which focuses on assessing the ameliorative effect of Anchomanes difformis (AD) on kidney nephrotoxicity induced with gentamicin in male Wistar rats.
Materials
Plants Materials: Anchomanes difformis root was gotten from Ede oballa, Nsukka, Enugu state of Nigeria. The plant was identified and authenticated by Mr Alfred Ozioko of the international centre for ethnomedicine and drug development Nsukka, Nigeria.
Experimental Animals: Adult rats of Swiss albino strain of both sexes weighing 123 g ± 147 g were obtained from the animal holding unit of the Department of Zoology and Environmental studies, University of Nigeria, were used for the study. The animals were housed under normal conditions of 25 ± 20C and 12h light/dark cycle). The rats were fed with standard pellets (Grand Cereals Ltd, Enugu, Nigeria) and had unlimited access to clean drinking water. The guide used for the care and use of laboratory animal procedures of this study were followed accordingly (Indian Council of Medical Research, 2001). Equipment: Centrifuge (Beckman Coulter Co., Indianapolis, Indiana, USA), Columns (Sigma-Aldrich Co., St. Louis, Missouri, USA), Micro-pipettes (Globe Scientific Inc., Mahwah, New Jersey, USA), Refrigerator (Thermo-cool Public Ltd Co., Ilupeju, Lagos, Nigeria), Rotary evaporator (Adams Equipment Inc., Oxford, England, UK), Spectrophotometer (Spectrum Laboratories, Stamford, Connecticut, USA), Electrolyte Analyzer (Spectrum Laboratories, Stamford, Connecticut, USA).
Chemicals and Reagents: All chemicals and reagents employed in this study were of analytical grade and were products of Sigma-Aldrich Co., St. Louis, Missouri, USA
Methods
Preparation of Plant Material: The Fresh rhizomes of Anchomanes difformis was collected and washed to remove dirt. The plant material was cut into pieces and shade-dried with regular turning to avoid decaying. The dried rhizome was pulverized into powdered form using a mechanical grinder. A known weight of the pulverized rhizome (400 g) was macerated in 700 ml absolute methanol using conical flask. The mixture was left for 48 hours; after which it was filtered into a flat-bottomed flask using a muslin cloth. The filtrate was air dried for 24 hours at room temperature to obtain the yield. Experimental Design: The study was done for 14 days and the animals were randomly divided into four groups as follows: group 1 (normal control) received 5 ml/kg/day normal saline; group 2 (positive control) received 5 ml/kg/day normal saline and 100 mg/kg/day gentamicin; group 3 received 150 mg/kg/day Anchomanes difformis and 100 mg/kg/day gentamicin; and group 4 received 300 mg/kg/ day Anchomanes difformis and 100 mg/kg/day gentamicin. Sample Collection: After 24 hours of the last administration, animals were sacrificed by ocular puncture and the kidneys were excised, trimmed of extraneous tissues and weighed. One of the kidneys was dissected and place in a container containing 10% formalin for histopathological examinations.
Phytochemical Screening of Methanol Extract of Anchomanes difformis: The phytochemical screening of the methanol extract of Anchomanes difformis was done according to standard method as described by Sofowora (1999), Harbone (1973) and Trease and Evans (1989).
Body Weight and Organ Weight Gain: The body weight gain was measured using a digital top scale balance while the kidneys of rats in the various groups were excised after blood collection. The organs were trimmed of extraneous tissues, placed on a gauze pad soaked with saline to retard desiccation and were weighed immediately (the paired organs were weighed together) and calculated for their standard organ weight.

Kidney Function Snalysis: Urea concentrations were analyzed using the GLDH method using the kit manufactured by Anamol Laboratories Private Ltd. Briefly, in a clean test tube, add 500μl of urea GLDH R1 solution, 500μl of urea GLDH R2 solution and 10μl of urine. Vortex vigorously and take your absorbance between 430 nm. Creatinine levels were analyzed using the kit manufactured by Anamol Laboratories Private Ltd. Briefly, in a clean test tube, add 500μl of creatinine picric solution, 500μl of creatinine diluent solution and 15μl of urine. Vortex vigorously and take your absorbance between 400 to 700 nm.
Electrolyte Analysis: The electrolyte analysis was carried out using an electronic electrolyte analyzer and the electrolyte measured were K+, Cl ̶ , Na+, CO2, Ca2+ and Anion gap. Briefly, blood sample were collected by ocular puncture of the Wister rats. The samples were put in a plain tube and spun using a centrifuge to obtain the serum. The serum was fed into the probe of the electrolyte analyzer and the concentration of the ions were displayed after 20 seconds.
Determination of the Enzymatic Antioxidants: Superoxide dismutase (SOD) activity was assayed by the method described by Xin et al. (1991). Briefly, adrenalin (0.01g) was dissolved in 17ml of distilled water and 0.1ml of serum and 0.9ml of phosphate buffer (pH 7.8) were taken in triplicates in 2.5ml buffer. A volume, (0.3ml) adrenaline solution was added and mixed inside the cuvette. The absorbance was taken at 480nm at 30 seconds interval for five (5) times The activity of catalase was assayed by the method of (Aebi, et al. [12]). Briefly, add 0.1ml of the test sample into a tube containing 0.9ml of hydrogen peroxide. Note, the ultra violet absorption of hydrogen peroxide can be easily measured at 240nm. On the decomposition of hydrogen peroxide (H2O2) by catalase the absorption decreases with time and from this decrease catalase activity can be measured. The Glutathione peroxidase assay was done according to the method of (Paglia, et al. [13]). Briefly, the sample tissue homogenate was added to a solution containing glutathione, glutathione reductase, and NADPH. The enzyme reaction was initiated by adding the substrate, hydrogen peroxide and the A340 and recorded. The rate of decrease in the A340 is directly proportional to the GPx activity in the sample.
Estimation of the Extent of Lipid Peroxidation (Malondialdehyde): The Determination of Lipid Peroxidation (Malondialdehyde) was done according to the method described by (Wallin, et al. [14]). A volume, 0.1ml of the serum was mixed with 0.9ml of H2O in a beaker. A volume, 0.5ml of 25% TCA (trichloroacetic acid) and 0.5ml of 1% TBA (thiobarbituric acid) in 0.3% NaOH were also added into the mixture. The mixture was boiled for 40 minutes in water-bath and then cooled in cold water. Then 0.1ml of 20% sodium dodecyl sulfate (SDS) was added to the cooled solution and mixed properly. The absorbance was taken at wavelength 532nm and 600 nm against a blank.

Histopathological Examination of the Organ: Histopathological examination was done according to the method described by (Drury, et al. [15]). After excision, the kidney was carefully dissected out from the abdominal region, fixed in 10% formalin solution for 72 hours and sliced into a thickness of 2.1 mm. The tissues were dehydrated with alcohol of graded concentrations and treated with paraffin wax and thereafter cast into blocks. Sections of the tissues were then cut using a microtome to 5μm. These were later attached to a slide and allowed to dry. The sample slides were subsequently stained in haematoxylin- eosin. Tissue sections of the organs collected from the animals in group 1 to 4 were prepared for histopathological examination using standard techniques. The slide sections were examined using Motic Light microscope and the photomicrographs were taken using Motic © microscope camera.
Statistical Analysis: The data obtained were analyzed statistically. The results were expressed as mean ± standard deviation (SDs). One-way analysis of variance (ANOVA) was used to analyze the data and mean difference p < 0.05 were considered significantly.
Phytochemical Result of Methanol Extract of Anchomanes Difformis
Table 1 shows the phytochemicals present in methanol extract of Anchomanes difformis. These phytochemicals include tannins, steroid, saponins, cardiac glycosides, alkaloid and flavonoid.
Note: + = Present, ++ = Moderately Present, +++ = Very much.
Effect of Methanol Extract of Anchomanes Difformis on the Organ Weight of Wistar Rats
Table 1 reveals the effect of Anchomanes difformis on the organ weight of the rats. There was no significant (p > 0.05) increase between group 2 (gentamicin control) and group 1 (normal control). Similarly, there was no significant (p > 0.05) decrease between the control and treatment groups.
Effect of Methanol Extract of Anchomanes Difformis on The Body Weight of Wistar Rats
Result from Table 2 shows a significant increase (p > 0.05) in the body weight of animals in the control group and treatment group after 14 days.
Note: Results are expressed in Means ± SD (n = 4) Mean values with different letters as superscripts down the columns are considered significant at p < 0.05.
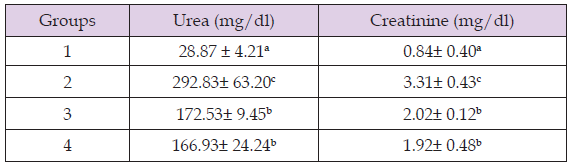
Effect of Methanol Extract of Anchomanes Difformis on Kidney Function Parameters of Wistar Rats
Table 3 reveals that there was significant increase in kidney function parameters of group 2 compared to group 1. However, treatment with the extract led to a significant decrease in kidney function parameters of group 3 and 4 compared to group 2.
Note: Results are expressed in Means ± SD (n = 4) Mean values with different letters as superscripts across the columns are considered significant at p < 0.05.
Effect of Methnol Extract of Anchomanes Difformis on Electrolyte Concentration of Wistar Rats
Table 4 shows that there was no significant difference (p < 0.05) in the K, Na, Cl, and Ca2+ concentrations of all the groups. However, there was a significant increase (p > 0.05) in CO2 concentration of treatment group 4 compared to group 3. Also, there was a significant increase (p > 0.05) in anion gap concentration of treatment group 3 compared to group 1 and 4.
Table 4: Effect of methanol extract of Anchomanes difformis on kidney function parameters of wistar rats.
Note: Results are expressed in Means ± SD (n = 4) Mean values with different letters as superscripts across the columns are considered significant at p < 0.05.
Effect of Methnol Extract of Anchomanes Difformis on Antioxidant Parameters of Wistar Rats
Table 5 shows there was a significant decrease in antioxidant parameters of group 2 compared to group 1 except for glutathione peroxidase. Furthermore, the result revealed that treatment with the extract significantly increased (p > 0.05) glutathione peroxidase and superoxide dismutase levels of group 3 and 4 compared to group 2. In the same vein, there was significant increase (p > 0.05) in catalase level of group 4 compared to group 2.
Effect of Methnol Extract of Anchomanes Difformis on Lipid Peroxidation of Wistar Rats
Table 6 reveals that there was significant increase in MDA levels of group 2 compared to group 1. However, treatment with the extract led to significant decrease in MDA levels of group 3 and 4 compared to group 2 Table 7.
Table 5: Effect of methanol extract of Anchomanes difformis on electrolyte concentration of wistar rats.
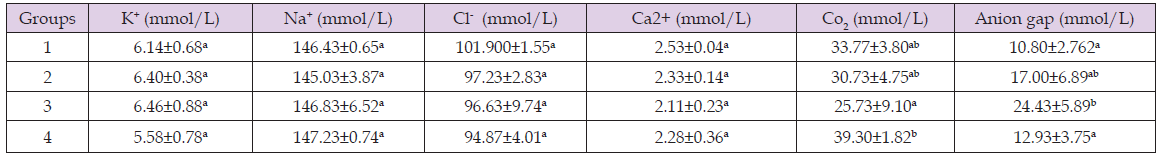
Note: Results are expressed in Means ± SD (n = 4) Mean values with different letters as superscripts across the columns are considered significant at p < 0.05.
Table 6: Effect of methanol extract of Anchomanes difformis on antioxidant parameters of wistar rats.
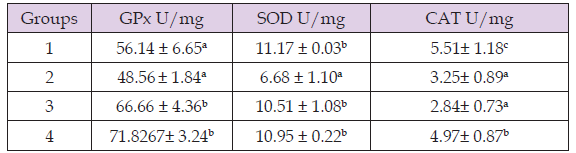
Note: Results are expressed in Means ± SD (n = 4) Mean values with different letters as superscripts across the columns are considered significant at p < 0.05.
Note: Results are expressed in Means ± SD (n = 4) Mean values with different letters as superscripts across the columns are considered significant at p < 0.05.
Histology of the Kidney After Administration of Anchomanes Difformis Extracts
The histologic result showed a photomicrograph of kidney section with a few shrink glomeruli (blue arrow) and minor (non-severe) homogenous tubular cast (star) observed within the renal cortex of the kidney (Figure 1). Photomicrograph of kidney section showed arterial media hypertrophy (blue arrow) characterized by circumferential thickening of the artery especially the tunica media. However, minor (non-severe) homogenous tubular cast (black arrow) was observed within the renal cortex of the kidney, usually associated with glomerular permeability an indication of onset of progressive nephropathy (Figure 2). The result further revealed a photomicrograph of kidney section with a severe homogenous tubular cast (black arrow) within the renal cortex of the kidney (Figures 3 & 4). showed a photomicrograph of kidney section of kidney of rat with intact tubules (black arrow) and widely dilated renal pelvis filled with cellular infiltrates (CI) associated with inflammation of the renal pelvis from the ascending lesions in the urinary bladder.
This study was conducted to know the effect of Anchomanes difformis methanol extract in ameliorating gentamicin induced kidney nephrotoxicity. First, Phytochemical screening of the plant was done to know the phytochemicals present in the plant extract. The result reveals the presence of flavonoid, cardiac glycosides, saponins, alkaloids and steroids. Among these phytochemicals, flavonoids and saponins have been reported to possess renal protective effects against numerous nephrotoxic agents that causes kidney damages (Vargas, et al. [16]). Next, gentamicin was administered to the experimental animals for fourteen days. After fourteen days of administration of gentamicin, there was significant increase in the levels of the kidney function parameters such as creatinine and urea. Increase in urea and creatinine levels could suggest a successful induction of nephrotoxicity (Udupa, et al. [1]). However, not all cases of increase in creatinine and urea levels are associated with nephrotoxicity (Salazar, et al. [17]). In order to confirm the successful induction of nephrotoxicity, histological studies were carried out and the result showed a morphologically damaged architecture of the kidney cells, which confirms a successful induction of nephrotoxicity (Udupa, et al. [1]). This result corroborates the results of (Priyamvada, et al. [2]) which also showed a successful induction of nephrotoxicity after fourteen days of administration of gentamicin at 100 mg/kg/day.
Next treatment with methanol extract of the leaves of Anchomanes difformis was administered at two different concentrations for 14 days. The result showed that after 14 days of the treatment, there was time dependent significant decrease in the creatinine and urea concentration of the treatment groups. Decrease in creatinine and urea concentrations suggest that treatment with methanol extract of the leaves of Anchomanes difformis ameliorated the nephrotoxicity induced by gentamicin (Fahmy, et al. [18]). Creatinine and urea are biochemical parameters used to check the functioning of the kidney (Salazar, et al. [17]). Increasingly high levels of these biochemical parameters are early diagnosis of poor kidney function and in the absence of timely intervention could lead to kidney damage (Balakumar, et al. [6]). Decrease in the levels of these biochemical parameters after treatment with methanol extract of the leaves of Anchomanes difformis suggests that the plant has phyto-therapeutic potentials in ameliorating kidney nephrotoxicity. This corroborates with the result of (Alabi, et al. [19]) who also reported decreased levels of creatinine and urea after treatment with Anchomanes difformis. However, creatinine and urea test are the first line test for diagnosing kidney nephrotoxicity; other confirmatory tests were carried out. These tests include, antioxidants test, electrolyte test, lipid peroxidation test and histopathological studies (Kishore, et al. [20]).
The result of the antioxidant test showed that treatment with methanol extract of the leaves of Anchomanes difformis significantly increased the antioxidant level. Oxidants are reactive molecules that are produced inside the body that react with other cellular molecules to destroy them (Fakhruddin, et al. [21]). When it does that, it leads to pathological changes of the organ with whose cells it reacted with; in this case, the kidney cells (Ishimoto, et al. [18]). Antioxidants are biological scavengers of oxidants (Lobo, et al. [22]). Therefore, increase in antioxidant levels suggests that the extract has the potential of curbing/scavenging the free radical/oxidant pool, thereby preventing further damages to the kidney cells and restoring the kidney cells back to its normal architecture. This could be associated to the presence of phytochemicals such as flavonoid which have been suggested in previous studies to have antioxidants properties (Brooks, et al. [23]). This result also corroborates the result of (Alabi, et al. [24]) who reported increased antioxidants levels after treatment with Anchomanes difformis. Next lipid peroxidation study was done, as a further confirmatory test to the antioxidant potentials of Anchomanes difformis. The result showed that administration of the methanol leaf extract of Anchomanes difformis led to significant decrease in malondialdehyde (MDA) levels of the treatment groups. Increase in MDA level is a major biochemical marker for lipid peroxidation (Alabi, et al. [24]).
Lipid peroxidation can disturb the assembly of the kidney cell membrane, causing changes in fluidity and permeability, alterations of ion transport and inhibition of metabolic processes (Matsunami, et al. [25]). It could further lead to DNA and mitochondria damage which will ultimately lead to kidney damage (Scibior, et al. [26]). Thus, the reduction in MDA levels after treatment with Anchomanes difformis shows the potential of Anchomanes difformis in ameliorating damages caused by lipid peroxidation after induction of nephrotoxicity with gentamicin in the experimental animals. This corroborates with the results of (Alabi, et al. [24]) who reported decrease in MDA levels after treatment with Anchomanes difformis. However, Anchomanes difformis did not have any effect on the electrolyte levels as there were no significant difference seen in all the groups. Finally, the histopathological study showed that induction of gentamicin damaged the morphological architecture of the kidney cells which was gradually ameliorated after 14 days of treatment with different concentration of methanol leaf extract of Anchomanes difformis (Alabi, et al. [19]). However, treatment with Anchomanes difformis did not completely reverse the histological damages caused by the induction of gentamicin in the experimental rats [27]. The mechanism behind this is not yet understood but could be associated to the presence of phytochemicals such as flavanols which has been shown by previous study to have potentials in ameliorating kidney damages (Brooks, et al. [23]) [28].
This study was carried out to know the effect of methanol leaf extract of Anchomanes difformis in ameliorating nephrotoxicity induced by gentamicin in experimental rats. The study showed that methanol leaf extract of Anchomanes difformis could possess phyto-therapeutic potentials in ameliorating nephrotoxicity induced by gentamicin.
The study was conducted in accordance to the regulations and approval of the Ethics and Biosafety Committee of the Faculty of Biological Sciences, University of Nigeria, Nsukka.
EG: conceptualized the work, supervised the work, validated it,
reviewed and edited it.
EC: contributed to data curation, formal analysis, investigation,
methodology and writing of the original draft.
MA: contributed to data curation, formal analysis, funding acquisition,
investigation, methodology, project administration and writing
of the original draft.
KC: contributed to the methodology, project administration and
writing of the original draft.
PN: contributed to data curation, formal analysis, funding acquisition,
investigation, methodology, project administration and writing
of the original draft.
PC: contributed to data curation, formal analysis, funding acquisition,
investigation, methodology, project administration and writing
of the original draft.
IM: contributed to data curation, formal analysis, funding acquisition,
investigation, methodology, project administration and writing
of the original draft.
All authors read and approved the final manuscript
All the authors gave their consent for the publication of the research.
The authors have no conflict of interest to declare.
No funding was received for this research.
The authors acknowledge the Department of Biochemistry, University of Nigeria, Nsukka for their contribution to the work.


