Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: May 23, 2024; Published: June 05, 2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy
DOI: 10.26717/BJSTR.2024.56.008918
Diclofenac, a phenylacetic acid derivative, is the most frequently used nonsteroidal anti-inflammatory drug in Europe. Diclofenac has analgesic, antipyretic, and anti-inflammatory activities and is used for longterm symptomatic treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, pain, primary dysmenorrhea, and acute migraine. The usual daily oral dose of diclofenac is 50 to 150 mg given in divided doses. A 1% topical gel, a topical solution, and a transdermal patch are available for short-term treatment of pain and a 3% gel formulation is indicated for topical treatment of actinic keratosis. Diclofenac produces adverse-effects (particularly gastrointestinal) in about 20% of patients. The efficacy and safely of diclofenac, the prophylaxis and treatment with diclofenac and the trials conducted with diclofenac have been reviewed. Diclofenac is hydroxylated into 4’-hydroxy-diclofenac by CYP2C9 and is glucuronidated by UGT2B7. The penetration of diclofenac sodium into human tissues has been studied following topical application and oral administration and the concentration of diclofenac sodium in skeletal muscle is higher following the topical application than oral administration. The pharmacokinetics of diclofenac sodium have been studied in healthy subjects following a single intravenous administration of 75 mg of diclofenac sodium and the elimination halflife of diclofenac sodium is about 1.3 hours. The toxicity of diclofenac has been studied in-vitro and diclofenac causes intestinal and hepatic toxicity. The aim of this study is to review diclofenac efficacy and safely, prophylaxis, treatment, and trials conducted with diclofenac, the metabolism and the pharmacokinetics of diclofenac, and the toxicity induced by diclofenac.
Keywords: Diclofenac; Efficacy-Safely; Metabolism; Pharmacokinetics; Prophylaxis; Tissue-Concentration; Toxicity; Treatment; and Trials
Diclofenac, a phenylacetic acid derivative, is the most frequently used nonsteroidal anti-inflammatory drug in Europe. Diclofenac has analgesic, antipyretic, and anti-inflammatory activities. Its potency is substantially greater than that of other nonsteroidal anti-inflammatory drugs [1].
Diclofenac is approved in the United States for long-term symptomatic treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, pain, primary dysmenorrhea, and acute migraine. Multiple oral formulations are available, providing a range of release times; the usual daily oral dosage is 50 to 150 mg, given in several divided doses. For acute pain such as migraine, a powdered form for dissolution in water and a solution for intravenous injection are available. Diclofenac also is available in combination with misoprostol a prostaglandin E analogue. This combination retains the efficacy of diclofenac while reducing the frequency of gastrointestinal ulcers and erosions. A 1% topical gel, a topical solution, and a transdermal patch are available for short-term treatment of pain due to minor strains, sprains, and bruises. A 3% gel formulation is indicated for topical treatment of actinic keratosis. In addition, an ophthalmic solution of diclofenac is available for treatment of postoperative inflammation following cataract extraction and for the temporary relief of pain and photophobia in patients undergoing corneal refractive surgery [1].
Diclofenac displays absorption, extensive protein binding, and an elimination half-life of 1 to 2 hours. The short elimination half-life makes it necessary to give doses of diclofenac considerably higher than would be required to inhibit the cycllooxiganase-2 fully at peak plasma concentrations to afford sustained cyclooxygenase inhibition throughout the dosing interval. Thus, both cyclooxygenases isoforms are inhibited for the first phase of the dosing interval. However, as plasma levels decrease, diclofenac behaves like a cycloxigenase-2 inhibitor in the later phase of the dosing interval. There is a substantial first-pass effect, such that only about 50% of diclofenac is available systemically. The drug accumulates in synovial fluid after oral administration which may explain why its duration pf therapeutic effect is considerably longer than its plasma elimination half-life. Diclofenac is metabolized in the liver by CYP2C9 to 4’-hydroxy-diclofenac, the principal metabolite, and is glucuronidated by UGT2B7 and the metabolites are excreted in the urine (65%) and in bile (35%) [1].
Diclofenac produces adverse-effects (particularly gastrointestinal) in about 20% of patients. The incidence of serious gastrointestinal adverse-effects, hypertension, and myocardial infarction is similar to the cyclooxygenase-2-selective inhibitors. Hypersensitivity reactions occurred following topical application and systemic administration. Severe liver injuries occur in 6 to 11 per 100,000 regular users annually. Elevation of hepatic transaminases in plasma by more than three times the upper normal limit, indicating liver damage, occurs in about 4% of patients. Transaminases should be monitored during the first 8 weeks of therapy with diclofenac. Other untoward responses to diclofenac include central nervous system effects, rashes, fluid retention, oedema, and renal function impairment. The drug is not recommended for children, nursing mothers, or pregnant women. Diclofenac is extensively metabolized. One metabolite, 4’-hydroxy-diclofenac, can form reactive benzoquinone imines (similar to acetaminophen’s metabolite) that deplete hepatic glutathione. UGT2B7 is the primary catalyst in the formation of another higher reactive metabolite, diclofenac acyl glucuronide. Genetic variation that causes higher catalytic activity of UGT2B7 is associated with an increased risk of hepatotoxicity among patients tacking diclofenac [1] (Figure 1).
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “diclofenac efficacy, safely”, “diclofenac prophylaxis”, “diclofenac treatment”, “diclofenac trials”, “diclofenac metabolism”, “diclofenac tissue concentration”, “diclofenac pharmacokinetics”, and “diclofenac toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Efficacy and Safely of Diclofenac
Comprehensive safety data from studies showed that diclofenac is safer and better tolerated than aspirin and is comparable in safety to ibuprofen and naproxen. Safety data from clinical trials showed that diclofenac administered at the daily dose of 150 mg caused lower-rate of adverse-reactions than other nonsteroidal anti-inflammatory drugs except for naproxen at 500 mg daily [2]. Diclofenac administered at the daily dose of 150 mg relieved pain more efficacious than ibuprofen administered at the daily dose of 1,200 [3]. Soft-gel capsules containing 100 mg of diclofenac sodium provided a rapid onset of analgesic activity, a prolonged analgesic duration, and were well-tolerated in patients undergoing the extraction of third molar tooth [4]. Diclofenac ophthalmic solution was a safe and effective analgesic in the treatment of traumatic corneal abrasions [5]. A daily dose of 150 mg of diclofenac is effective as 2,400 mg daily of ibuprofen and as 3,600 mg daily of aspirin in treatment of pain [6]. Diclofenac epolamine 1.3% topical patch relieved acute pain from soft-tissue injury and was well-tolerated [7]. Topical diclofenac diethylamine 2.32% gel applied twice-daily relieved pain, improved function, and reduced symptomatic healing time in uncomplicated ankle sprain, and was well-tolerated [8].
Prophylaxis with Diclofenac
Diclofenac administered at the daily dose of 75 to 150 mg for 9 to 42 days effectively prevents heterotopic ossification and can be used routinely after total hip arthroplasty [9]. A total of 245 patients undergoing total hip arthroplasty received 150 mg of diclofenac twice-daily for 7 days and this treatment prevented heterotopic ossification [10]. Forty-one patients received 50 mg thrice-daily of diclofenac for 7 days after being discharged for a colic episode. Short-term of diclofenac prophylaxis effectively prevented new colic episodes and significantly reduced (P-value < 0.05) the number of hospital readmissions [11]. A total of 100 patients received diclofenac at the daily dose of 150 mg for postoperative analgesia after cardiac surgery. Diclofenac relieved postoperative pain and prevented complications in patients undergoing cardiac surgery [12]. A rectal dose of 25 mg of diclofenac was administered to 276 patients aged over 75 years. Diclofenac effectively and safely prevented post-endoscopic retrograde cholangiopancreatography pancreatitis in these patients [13]. One-hundred-fifty patients with acute pancreatitis received diclofenac intramuscularly at the daily dose of 75 mg or received diclofenac rectally at the daily dose of 100 mg. Prophylaxis with diclofenac given intramuscularly or rectally effectively managed post-endoscopic retrograde cholangiopancreatography pancreatitis [14]. Prophylaxis with 100 mg daily of diclofenac administered rectally to 346 patients reduced the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis [15]. Eight patients received diclofenac intramuscularly at the dose of 75 mg followed by the infusion of 5 to 10 ml/kg per hour of isotonic saline over 4 hours after endoscopic retrograde cholangiopancreatography pancreatitis or received 500 ml of isotonic saline plus placebo. Intramuscular diclofenac and fluid replacement lowered the rate of pancreatitis more effectively (P-value = 0.047) that saline plus placebo [16].
Treatment with Diclofenac
Diclofenac administered at the daily dose of 150 mg demonstrated high efficacy in treatment of pain, physical disability, and rheumatic disease [17]. Thirty-five nulliparous women with painful symptoms of primary dysmenorrhea received diclofenac sodium at the daily dose of 75 mg and this treatment effectively reduced pain and bleeding at menstruation [18]. The efficacy of diclofenac sodium was compared to that of hyoscine butylbromide, a spasmolytic agent, in the treatment of 78 patients with ureteral colic. Forty-two patients (53.8%) received diclofenac sodium intramuscularly and 36 patients (46.2%) received hyoscine butylbromide intramuscularly. Diclofenac sodium was more effective (P-value < 0.05) than hyoscine butylbromide in treating ureteral colic and had fewer adverse-effects [19]. One-hundred-thirty-nine patients were enrolled and 69 patients (49.7%) received diclofenac at the dose of 75 mg twice-daily and 70 patients (50.3%) received aspirin at the dose of 1,200 mg thrice-daily and fitness was significantly (P-value = 0.025) shorter in patients who received diclofenac [20]. Topical 0.1% diclofenac applied topically is superior (P-value = 0.02) to 0.1% dexamethasone applied topically in treatment of subconjunctival haemorrhage in patients who underwent surgery of the left eye [21]. Diclofenac sodium 0.1% applied topically is as effective as dexamethasone phosphate 1% applied topically in treatment of postoperative inflammation in in patients who underwent cataract surgery [22].
Trials Conducted with Diclofenac
Two multicentre, double-blind trials compared the efficacy and safely of diclofenac administered at the daily dose of 150 mg to 130 patients to those of aspirin administered at the daily dose of 3,600 mg to 194 patients (Study A) and to those of naproxen administered at the daily dose of 1,000 mg to 223 patients (Study B) and all patients had rheumatoid arthritis. Diclofenac, aspirin, and naproxen produced significant improvement (P-value ≤ 0.01) of rheumatoid arthritis symptoms from baseline. Patients who received diclofenac experienced fewer adverse-effects (P-value < 0.05) than patients who received aspirin or naproxen. In study A, significantly fewer (P-value < 0.05) patients who received diclofenac discontinued the treatment for tinnitus and deafness compared to patients who received aspirin. In study B, fewer patients (P-value < 0.05) who received diclofenac discontinued the treatment due to adverse-effects compared to patients who received naproxen. Diclofenac, aspirin, and naproxen demonstrated similar efficacy and diclofenac was better tolerated than either aspirin or naproxen [23]. A six-week, double-blind, randomized, multicentre, clinical trial compared the efficacy and safely of diclofenac sodium administered at the daily dose of 150 mg to those of placebo administered to 182 patients and all patients had rheumatoid arthritis. A greater improvement (P-value < 0.05) of rheumatoid arthritis symptoms was observed in patients who received diclofenac. Fewer patients (P-value < 0.05) who received diclofenac discontinued the treatment because of lack of therapeutic response. Adverse-effects were observed in 28.1% patients who received diclofenac and in 21.0% patients who received the placebo. Diclofenac was found to be more effective and safer than placebo and was well-tolerated in treatment of patients with rheumatoid arthritis [24]. In an open, comparative, multicentre trial, 96 patients with arthritis of the large joints received 75 mg diclofenac once-daily, or 50 mg of diclofenac twice-daily, or 250 mg twice-daily of naproxen and the treatments lasted 14 days. The length of history of pain was longer (P-value < 0.05) in patients who received naproxen. Clinical parameters showed that the best degree of improvement was obtained with diclofenac administered at the dose of 50 mg twice-daily and diclofenac had better tolerability than naproxen [25]. A randomized, open-label, parallel, active, controlled, clinical trial was conducted in 139 patients with osteoarthritis of the knee who randomly received either a curcumin administered to the dose of 500 mg thrice-daily or diclofenac administered at the dose of 50 mg twice-daily and treatments lasted 28 days. Curcumin had similar efficacy as diclofenac and was better tolerated [26]. A double-blind, randomised, multicentre trial assessed the efficacy and safely of diclofenac administered at the dose of 150 or 75 mg once-daily in dual release capsules compared to enteric coated tablets administered at the dose of 50 mg thrice-daily and all patients had osteoarthritis. Pain relief was the main treatment target. Efficacy was observed in all treatments and the overall safely and tolerability were good. For the dose of 75 mg once-daily of diclofenac a lower incidence of liver and biliary adverse-effects was reported. Considering the efficacy and safely, the once-daily administration of diclofenac at the dose of 75 mg in dual release capsules is the appropriate dosage regimen for mid- and long-term treatment of osteoarthritis [27]. A phase III, multicentre, randomized, double-blind, placebo-controlled trial was conducted in 440 patients who randomly received either 30 mg of diclofenac etalhyaluronate (N = 220) or placebo (N = 220) intravenously once-every 4 weeks for 20 weeks (a total of 6 injections) and patients were followed up for 24 weeks. The primary endpoint was the change of pain score from baseline. At 12 weeks of treatment, subjects who received diclofenac etalhyaluronate showed significant improvement of pain from baseline (P-value < 0.001) and a significant reduction (P-value < 0.001) of pain was observed as early as week 1 of treatment. Diclofenac etalhyaluronate resulted in significant improvement in pain compared to placebo [28]. A prospective, open-label, phase IV, clinical trial was conducted in 182 patients with mild, moderate, or acute musculoskeletal pain. The objectives of treatment were the control of pain 3 days after dosing, the evaluation of the analgesic effect, the duration of pain relief, and the tolerability of treatment. Patients took ≥ 1 capsule of diclofenac epolamine 12.5 mg and the most common musculoskeletal conditions were arthralgia (39.0%) and low back pain (23.1%). The onset of analgesic effect was rapid, the complete pain relief was reached after a mean of 49.5 min, the overall treatment satisfaction occurred in 92.9% of patients, and the treatment was well-tolerated. The low-dose of 12.5 mg of diclofenac epolamine capsules formulation exerted rapid, effective, and safe analgesic activity in patients with mild, moderate, or acute musculoskeletal pain, and the treatment satisfaction occurred in more than 90% of patients [29]. A double-blind, clinical trial compared the efficacy of diclofenac sodium administered intramuscularly at the daily dose of 100 mg (N = 20) to that of placebo (N = 20) and patients had three consecutive migraine attacks. If migraine still remained after 6 hours of treatment the patients also received 100 mg diclofenac sodium suppository. Diclofenac sodium was more effective than placebo (P-value < 0.01) in treating migraine [30]. Two 12-week, double-blind, double-dummy, randomized, multicentre trials compared the safely and efficacy profiles of diclofenac topical solution to those of oral diclofenac in treatment of osteoarthritis of the knee. The most common adverse-effect was the dry skin which occurred in 24.1% of patients who received diclofenac topically and in 1.9% of patients who received diclofenac orally (P-value < 0.0001). Fewer gastrointestinal (25.4% versus 39.0%; P-value < 0.0001) and fewer cardiovascular (1.5% versus 3.5%; P-value = 0.055) occurred in patients who received diclofenac topically and orally, respectively. These results indicate that diclofenac administered topically represents a useful alternative to oral diclofenac in treatment of osteoarthritis of the knee [31]. A randomized, double-blind, double-dummy, equivalence trial compared the safely and efficacy of topical diclofenac solution to those of oral diclofenac in relieving pain caused by osteoarthritis of the knee. A total of 622 patients with osteoarthritis of the knee randomly received either a topical solution of diclofenac or capsules containing 50 mg of diclofenac. Patients applied 50 drops of diclofenac solution or took 1 capsule of diclofenac and both treatments were given thrice-daily for 12 weeks. Safely was assessed by evaluation of adverse-effects, vital signs, and laboratory data. The topical application of diclofenac produced relief of symptoms equivalent to oral diclofenac in patients with osteoarthritis of the knee. Topical application of diclofenac caused minor local skin irritation, lower incidence of gastrointestinal adverse-effects, and less abnormal laboratory values [32].
Metabolism of Diclofenac
(Tang, et al. [33]) studied the metabolism of diclofenac in-vitro using human liver microsomes. The metabolism of diclofenac in humans partitions between acyl glucuronidation and phenyl hydroxylation, with the former reaction catalysed primarily by UGT2B7 while the latter is catalysed by CYP2C9. The 4’-hydroxylation of diclofenac represents a feature reaction for CYP2C9 catalysis, and this regioselective oxidation is presumably dictated by interactions of the carboxylate moiety of the substrate with a putative cationic residue of the enzyme. Lazarska et al. [34] observed that the possession of the UGT2B7*2 and CYP2C8*4 alleles are responsible of diclofenac-induced liver injury. 4’Hydroxy-diclofenac can form reactive benzoquinone imines that deplete hepatic glutathione. UGT2B7 catalyses the formation of another reactive metabolite: the diclofenac acyl glucuronide (Figures 2 & 3).
Penetration of Diclofenac into Human Tissues
Seefried et al. [35] studied the penetration of diclofenac diethylamine into the synovial tissue and fluid of osteoarthritic knee. Thirty patients received topical diclofenac diethylamine gel 2.32% w/w 4 grams twice-daily. Table 1 shows the concentration of diclofenac diethylamine in synovial tissue, in synovial fluid, and in plasma. (Miyatake et al. [36]) administered either a capsule containing 37.5 mg of diclofenac sodium (N = 7) or two tapes containing a total of 30 mg of diclofenac sodium (N = 7) to patients who were scheduled to undergo knee arthroplasty due to osteoarthritis. At 12 hours after prescription, the diclofenac sodium concentration was measured in the fat, muscle, synovial membrane, plasma, and synovial fluid. Table 2 shows the concentration of diclofenac sodium in fat, muscle, synovial membrane, plasma, and synovial fluid. Brunner et al. [37] studied the relative bioavailability of diclofenac in plasma, subcutaneous adipose tissue, and skeletal muscle after repeated topical application of a spry gel containing 4% diclofenac and after the administration of enteric coated tablets containing 50 mg of diclofenac. Diclofenac (48 mg) was applied topically thrice-daily for 3 days onto a defined area of the thigh of 12 healthy males. After a 14-day wash out period, the subjects received diclofenac orally at the dose of 50 mg thrice-daily for 3 days. In-vivo microanalysis in subcutaneous tissue and in skeletal muscle was performed immediately after the final dose of both treatments on day 4 and 48 hours later and plasma and tissue specimens were sampled simultaneously. Table 3 shows the area under the concentration- time curve and the peak concentration of diclofenac in plasma, in subcutaneous tissue, and in skeletal muscle.
Table 1: Concentration of diclofenac diethylamine in synovial tissue, in synovial fluid, and in plasma, by (Seefried et al. [35]).
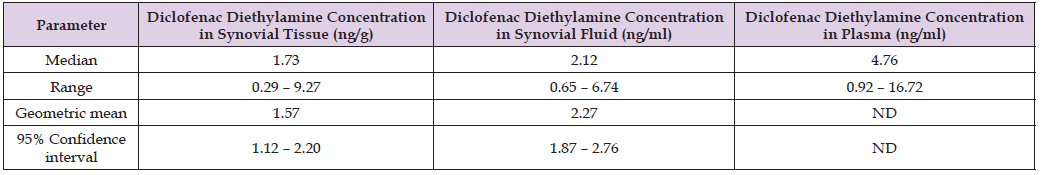
Note: ND = not determined.
This table shows that the concentration of diclofenac diethylamine in synovial tissue and in synovial fluid is lower than that in plasma.
Table 2: Concentration of diclofenac sodium in fat, skeletal muscle, synovial membrane, plasma, and synovial fluid which have been obtained in 7 patients who received a capsule containing 37.5 mg of diclofenac sodium and in 7 patients who received two taps containing a total of 30 mg of diclofenac sodium, by (Miyatake et al. [36]).
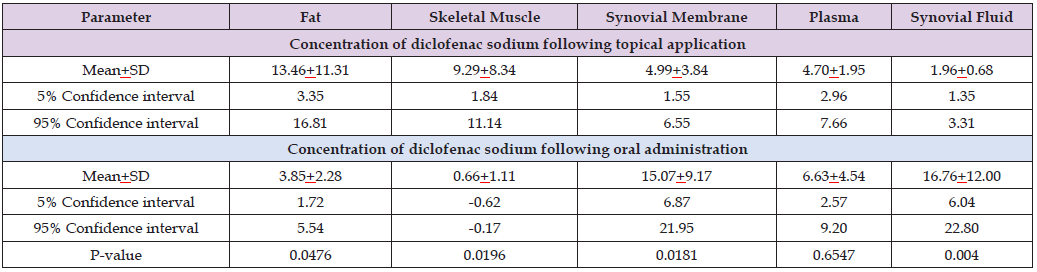
Note: This table shows that the concentration of diclofenac sodium in fat and in skeletal muscle is higher following the topical application, the concentration of diclofenac sodium in synovial membrane and in synovial fluid is higher following the oral administration, and the concentration of diclofenac sodium in plasma is similar following the topical application and the oral administration. As the concentration of diclofenac sodium in skeletal muscle is higher following the topical application the topical application is the preferred formulation of diclofenac sodium.
Table 3: Pharmacokinetic parameters of diclofenac which have been obtained in plasma (total drug) and subcutaneous tissue and skeletal muscle (free drug) of the thigh of 12 healthy males after the final dose of a 3 day multiple dose regimen of either topical application of diclofenac spry gel 4% or oral diclofenac 50 mg enteric coated tablets. Values are the median and 95% confidence interval, by (Brunner et al. [37]).
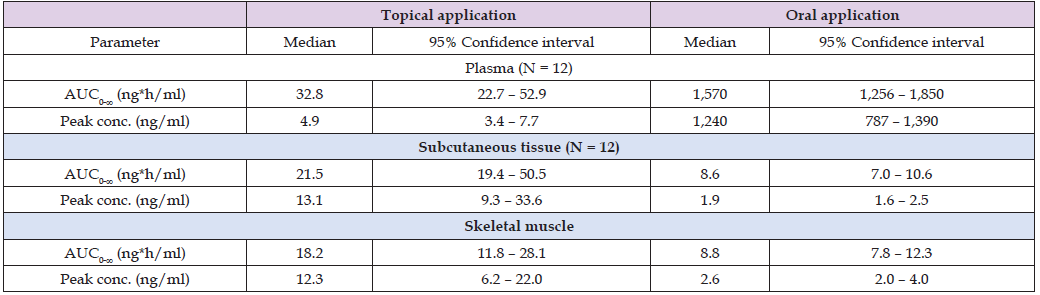
Note: AUC = area under the concentration-time curve.
This table shows that following topical application the area under the concentration-time curve of diclofenac is about double in plasma than in subcutaneous tissue and in skeletal muscle. The peak concentration of diclofenac in subcutaneous tissue and in the skeletal muscle is about 2.5 times higher than that in plasma. Following oral administration the area under the concentration-time curve of diclofenac is about 200 times higher in plasma than in subcutaneous tissue and in skeletal muscle and the peak concentration of diclofenac is about 500 times higher in plasma than that in subcutaneous tissue and skeletal muscle. Both the area under the concentration-time curve and the peak concentration of diclofenac in muscle are higher following topical application thus the topical application of is the preferred formulation of diclofenac.
Pharmacokinetics of Diclofenac Sodium
Leuratti et al. [38] studied the pharmacokinetics of diclofenac sodium in 17 healthy women and in 18 healthy men and a single intravenous of 75 mg of diclofenac sodium was administered to all women and men. Table 4 provides the vital data of the women and men included in the study, Table 5 summarizes the pharmacokinetic parameters of diclofenac sodium, and Table 6 summarizes the pharmacokinetic parameters of diclofenac sodium obtained following a bolus intravenous injection, following intramuscular injection, and following intravenous infusion of diclofenac sodium (Table 7).
Table 4: Vital data of the 17 healthy women and 18 healthy men included in the study. Values are the mean+SD and range, by (Leuratti et al. [38]).
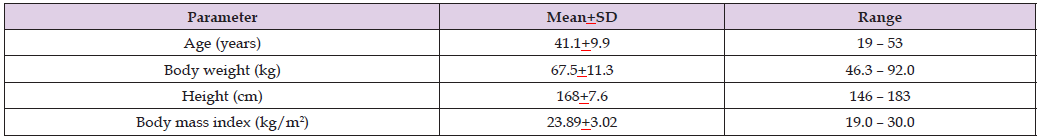
Table 5: Pharmacokinetic parameters of diclofenac sodium which have been obtained in 8 healthy women and healthy men following the single intravenous administration of 75 mg of diclofenac sodium injected in 5 seconds, in 15 seconds, or in 30 seconds. Values are the mean+SD or the median and (range), by (Leuratti et al. [38]).
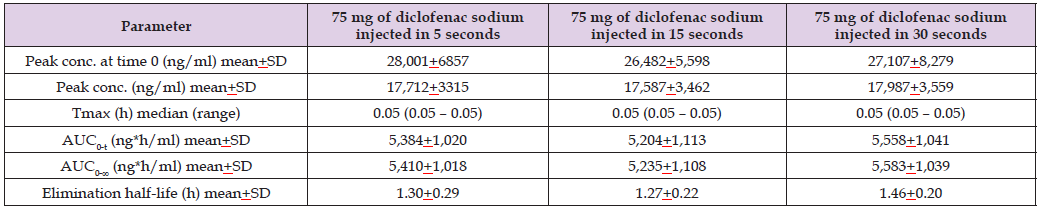
Note: Tmax = time to reach the peak concentration. AUC0-t = area under the concentration-time curve from time 0 to the last observed concentration time.
AUC0-∞ = area under the concentration-time curve from time 0 to infinity.
This table shows that the pharmacokinetic parameters are similar according to the three lengths of injection. Diclofenac sodium is eliminated rapidly as the
elimination half-life is about 1.3 hours. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability
is accounted by a wide variation in the vital data of the subjects included in the study.
Table 6: Pharmacokinetic parameters of diclofenac sodium which have been obtained in 18 healthy women and healthy men following a single intravenous bolus of 75 mg/1 ml solution of diclofenac sodium, following an intramuscular injection of 75 mg/3 ml of solution of diclofenac sodium, and following the 30 min of intravenous infusion of 75 mg/3 ml of solution of diclofenac sodium. Values are the mean+SD or the median and (range), by (Leuratti et al. [38]).
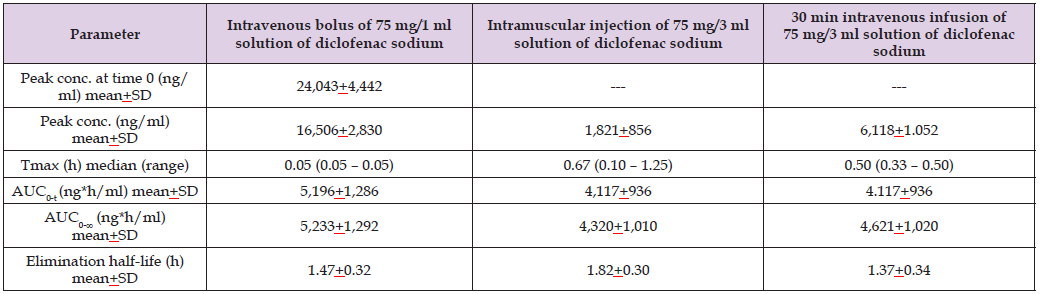
Note: Tmax = time to reach the peak concentration. AUC0-t = area under the concentration-time curve from time 0 to the last observed concentration time.
AUC0-∞ = area under the concentration-time curve from time 0 to infinity.
This table shows that, except for the peak concentration, the pharmacokinetic parameters of diclofenac sodium are similar according to the three formulations
of diclofenac sodium. The peak concentration of diclofenac sodium is higher following the intravenous bolus injection than following the intramuscular
injection and the intravenous infusion of diclofenac sodium suggesting that diclofenac sodium diffuses in the body more rapidly following the
intravenous bolus injection than following the intramuscular injection and the intravenous infusion.
Table 7:Ratio of the concentration of diclofenac diethylamine in synovial tissue to that in plasma and the ratio of the concentration of diclofenac diethylamine in synovial fluid to that in plasma, by (Seefried et al. [35]). Table 2 shows the ratio of synovial tissue concentration to plasma concentration of diclofenac diethylamine and the ratio of synovial fluid concentration to plasma concentration of diclofenac diethylamine.

Note: This table shows that the concentration of diclofenac diethylamine in synovial tissue is about one-fourth of that in plasma and the concentration of diclofenac diethylamine in synovial fluid is about one-half of that in plasma.
All Subjects Were White
Toxicity Induced by Diclofenac
The use of diclofenac is associated with a high prevalence of gastrointestinal and hepatic adverse-effects. (Niu et al. [39]) incubated precision-cut jejunum slices obtained from 18 human donors with 600 μM and 400 μM diclofenac up to 24 hours. The toxicity induced by diclofenac was demonstrated by ATP depletion, morphological damage, and lactate dehydrogenase leakage and it was greater following the inclusion in the incubation medium of 600 μM than 400 μM diclofenac. The main metabolite produced was 4’-hydroxy-diclofenac and the addition in the incubation medium of inhibitors of cytochrome P-450 such as ketoconazole or cimetidine decreased the formation of metabolites, reduced the consumption of diclofenac, with consequent increase of toxicity. These results suggest that diclofenac can induce toxicity in slices of human jejunum at therapeutically relevant concentrations thus diclofenac can induce toxicity in-vivo in humans. (Bort et al. [40]) stated that diclofenac causes adverse-effects in human liver. To assess the hepatotoxicity induced by diclofenac it was examined the effects of diclofenac on ATP concentration and on the impairment of ATP synthesis by mitochondria. Acute toxicity induced by diclofenac in human hepatocytes was preluded by a decrease in ATP concentration and impairment of ATP synthesis by mitochondria. Toxicity was also studied by adding the cytochrome P-450 inhibitors proadifen or ketoconazole to the incubation medium. These compounds reduced the metabolism of diclofenac with consequent reduction of diclofenac consumption and increase of hepatotoxicity. These results suggest that the toxic effect of diclofenac on human hepatocytes is caused by drug-induced decrease of ATP concentration and by impairment of ATP synthesis together with a futile consumption of NADPH. Tateishi et al. [41] stated that diclofenac is associated with severe liver injury and the adverse-effects are caused by reactive quinone imine and acyl glucuronide metabolites that deplete hepatic glutathione.
Diclofenac, a phenylacetic acid derivative, is the most frequently used nonsteroidal anti-inflammatory drug in Europe and diclofenac has analgesic, antipyretic, and anti-inflammatory activities. Diclofenac is used for long-term symptomatic treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, pain, primary dysmenorrhea, and acute migraine. The usual daily oral dose of diclofenac is 50 to 150 mg given in several doses. A 1% topical gel, a topical solution, and a transdermal patch formulation of diclofenac are available for shortterm treatment of pain due to minor strains, sprains, and bruises. A 3% gel formulation of diclofenac is indicated for topical treatment of actinic keratosis and an ophthalmic solution of diclofenac is available for treatment of postoperative inflammation following corneal refractive surgery. The elimination half-life of diclofenac is about 1.3 hours and the short elimination half-life makes it necessary to give doses of diclofenac considerably higher than would be required to inhibit the cyclooxygenase-2 fully at peak plasma concentrations to afford sustained cyclooxygenase inhibition throughout the dose interval. There is a substantial first-pass effect such that only about 50% of diclofenac is available systemically. Diclofenac induces adverse-effects (particularly gastrointestinal) in about 20% of patients. Hypersensitivity reactions occurred following topical application and systemic administration of diclofenac. Severe liver injuries occur in 6 to 11 per 100,000 regular users annually and the elevation of hepatic transaminases in plasma by more than three times the upper normal limit occurs in about 4% of patients who received diclofenac.
Diclofenac is hydroxylated into 4’-hydroxy-diclofenac which can form reactive benzoquinone imines that deplete hepatic glutathione [1]. The efficacy and safely of diclofenac have been reviewed. Diclofenac is safer and better tolerated than aspirin and has similar safely as ibuprofen and naproxen. Diclofenac administered at the daily dose of 150 mg has lower-rate of adverse-reactions than other nonsteroidal anti-inflammatory drugs except for naproxen administered at the daily dose of 500 mg [2], diclofenac administered at the daily dose of 150 mg reliefs pain more efficaciously than ibuprofen administered at the daily dose of 1,200 mg [3], in patients undergoing the extraction of third molar tooth, diclofenac sodium soft-gel of 100 mg provides a rapid onset of analgesic activity, prolongs analgesic duration, and is well-tolerated [4], diclofenac ophthalmic solution effectively and safely treats traumatic corneal abrasions [5], a daily dose of 150 mg of diclofenac is effective as 2,400 mg daily of ibuprofen and as 3,600 mg daily of aspirin in treatment of pain [6], diclofenac epolamine 1.3% topical patch reliefs acute pain from soft-tissue injury and is well-tolerated [7], and topical diclofenac diethylamine 2.32% gel applied twice-daily reliefs pain, improves function, reduces symptomatic healing time in uncomplicated ankle sprain, and is well-tolerated [8]. These results indicate that diclofenac administered at the daily dose of 150 mg has lower-rate of adverse-reactions than other nonsteroidal anti-inflammatory drugs except for naproxen administered at the daily dose of 500 mg, diclofenac sodium soft-gel of 100 mg provides a rapid onset of analgesia, prolongs analgesic duration, and is well-tolerated in patients undergoing the extraction of the third molar tooth, diclofenac ophthalmic solution effectively and safely treats traumatic corneal abrasions, a daily dose of 150 mg of diclofenac is effective as ibuprofen administered at the daily dose of 2,400 mg and as aspirin administered at the daily dose of 3,600 mg in treating pain, and diclofenac diethylamine 2.32% gel applied twice-daily reliefs pain, improves function, reduces symptomatic healing time in uncomplicated ankle sprain. The prophylaxis with diclofenac has been reviewed. Diclofenac administered at the daily dose of 75 to 150 mg for 9 to 42 days effectively prevents heterotopic ossification and can be used routinely after total hip arthroplasty [9], diclofenac administered at the dose of 150 mg twice-daily for 7 days prevents heterotopic ossification in patients who underwent total hip arthroplasty [10], diclofenac administered at the dose of 50 mg thrice-daily for 7 days to patients who were discharged for a colic episode prevents new colic episodes and reduces (P-value < 0.05) the number of hospital readmission [11], diclofenac administered at the daily dose of 150 mg reliefs pain and prevents complications in patients undergoing cardiac surgery [12], a rectal dose of 25 mg of diclofenac prevents post-endoscopic retrograde cholangiopancreatography pancreatitis [13], an intramuscular daily dose of 75 mg of diclofenac or a rectal daily dose of 100 mg of diclofenac prevents post-endoscopic retrograde cholangiopancreatography pancreatitis [14], a rectal dose of 100 mg of diclofenac prevents post-endoscopic retrograde cholangiopancreatography pancreatitis [15], an intramuscular dose of 75 mg of diclofenac followed by an infusion of isotonic saline prevents pancreatitis more effectively (P-value = 0.047) than saline plus placebo [16].
These results indicate that diclofenac prevents heterotopic ossification, prevents colic episodes and reduces the number of hospital readmission, diclofenac reliefs pain and prevents complications in patients who underwent cardiac surgery, prevents post-endoscopic retrograde cholangiopancreatography pancreatitis, and prevents pancreatitis more effectively than placebo. The treatment with diclofenac has been reviewed. Diclofenac administered at the daily dose of 150 mg treats pain, physical disability, and rheumatic disease [17], nulliparous women with painful primary dysmenorrhea received diclofenac sodium at the daily dose of 75 mg and this treatment reduces the pain and blending at menstruation [18], diclofenac sodium is more effective (P-value < 0.05) than hyoscine butylbromide in treating patients with ureteral colic and has fewer adverse-effects [19], diclofenac administered at the dose of 75 mg twice-daily shortens fitness more effectively (P-value = 0.025) than aspirin administered at the dose of 1,200 mg thrice-daily [20], 0.1% topic diclofenac is superior (P-value = 0.02) to 0.1% topic dexamethasone in treating subconjunctival haemorrhage in patients who underwent eye surgery [21], and diclofenac sodium 0.1% applied topically is as effective as dexamethasone phosphate 1% applied topically in treatment of postoperative inflammation in patients who underwent cataract surgery [22]. These results indicate that diclofenac effectively treats pain, physical disability, and rheumatic disease, reduces pain and blending in nulliparous women at menstruation, diclofenac is more efficacious than hyoscine butylbromide in treating ureteral colic, diclofenac administered at the dose of 75 mg twice-daily shortens fitness more effectively than aspirin administered at the dose of 1,200 mg thrice-daily, 0.1% topic diclofenac is superior to 0.1% topic dexamethasone in treating subconjunctival haemorrhage in patients who underwent eye surgery, and 0.1% topic diclofenac is effective as dexamethasone phosphate 1% applied topically in treatment of cataract surgery. The trials conducted with diclofenac have been reviewed. Two multicentre, double-blind trials compared the efficacy and safely of diclofenac administered at the daily dose of 150 mg to those of aspirin administered at the daily dose of 3,600 mg and to those of naproxen administered at the daily dose of 1,000 mg in patients with rheumatoid arthritis. Diclofenac, aspirin, and naproxen improve symptoms of rheumatoid arthritis and diclofenac is better tolerated than aspirin and naproxen [23], a sixweek, double-blind, randomized, multicentre, clinical trial compared the efficacy and safely of diclofenac sodium administered at the daily dose of 150 mg to those of placebo in patients with rheumatoid arthritis. Fewer (P-value < 0.05) adverse-effects occurred in patients who received diclofenac sodium and diclofenac sodium is more effective (P-value < 0.05) and safer than placebo in treatment of patients with rheumatoid arthritis [24], an open, comparative, multicentre trial assessed the efficacy of 75 mg once-daily of diclofenac, versus that 50 mg of twice-daily of diclofenac, or versus that of 250 mg twice-daily of naproxen in patients with arthritis of large joints and the treatments lasted 14 days. The best degree of improvements is obtained with diclofenac administered at the dose of 50 mg twice-daily and diclofenac is better tolerated than naproxen [25], a randomized, open-label, parallel, active, controlled, clinical trial compared the efficacy of curcumin administered at the dose of 500 mg thrice-daily to that of diclofenac administered at the dose of 50 mg twice-daily and treatments lasted 28 days. Patients had osteoarthritis and curcumin has similar efficacy as diclofenac in treating osteoarthritis and is better tolerated [26], a double-blind, randomized, multicentre trial assessed the efficacy and safely of dual release capsules containing 150 or 75 mg of diclofenac administered once-daily compared to enteric coated tablets containing 50 mg of diclofenac administered trice-dally. These formulations of diclofenac were administered to patients with osteoarthritis and the pain relief was the main treatment target. All treatments relief pain and the lower incidence of liver and biliary adverse-effects is observed with the administration of dual release capsules containing 75 mg of diclofenac administered once-daily. The administration of diclofenac at the dose of 75 mg once-daily in dual release capsules is the appropriate regimen for mid- and long-term treatment of osteoarthritis [27], a phase III, multicentre, randomized, double-blind, placebo-controlled trial compared the efficacy of diclofenac etalhyaluronate administered intravenously at the dose of 30 mg once-every 4 weeks for 20 weeks (a total of 6 injections) to that of placebo in relieving pain. At 12 weeks of treatment, diclofenac etalhyaluronate reliefs pain more effective (P-value < 0.001) than placebo and a reduction of pain (P-value < 0.001) is also observed as early as week 1 of treatment. Diclofenac etalhyaluronate reliefs pain more effectively than placebo [28], a prospective, open-label, phase IV, clinical trial assessed the analgesic effect of diclofenac epolamine in patients with musculoskeletal arthralgia and back pain. Patients received ≥ 1 capsule containing 12.5 mg of diclofenac epolamine and the onset of analgesic effect was rapid, complete pain relief occurred in a mean of 49.5 min, the overall treatment satisfaction occurred in 92.9% of patents and the treatment was well-tolerated. Low-dose of diclofenac epolamine produces rapid, effective, and safe analgesic activity in patients with musculoskeletal arthralgia and back pain [29], a double-blind, clinical trial compared the efficacy of diclofenac sodium administered intramuscularly at the dose of 100 mg to that of placebo in patients with migraine. Diclofenac sodium is more effective (P-value < 0.01) than placebo in treating migraine [30], two 12-week, double-blind, double-dummy, randomized, multicentre trials compared the efficacy and safely of diclofenac topical solution to those of oral diclofenac in treatment of osteoarthritis of the knee. The dry skin was the main adverse-effect which occurred in 24.1% of patients who received diclofenac topically and in 1.9% of patients who received diclofenac orally (P-value < 0.0001). Fewer gastrointestinal (P-value < 0.0001) and fewer cardiovascular (P-value = 0.055) adverse-effects occurred in patients who received diclofenac topically. Topical diclofenac is a useful alternative in treatment of osteoarthritis of the knee [31], a randomized, double-blind, double-dummy, equivalence trial compared the safely and efficacy of topical diclofenac solution to those of oral diclofenac in relieving pain due to osteoarthritis of the knee. Patients received 50 drops of diclofenac solution or 1 capsule of containing 50 mg of diclofenac and both treatments were given thrice-daily for 12 weeks. Topical application of diclofenac causes minor local skin irritation, lower incidence of gastrointestinal adverse-effects, and less abnormal laboratory values in patients with osteoarthritis of the knee [32]. The metabolism of diclofenac has been reviewed. Diclofenac is hydroxylated into 4’-hydroxy-diclofenac by CYP2C9 and is glucuronidated by UGT2B7 [33] and UGT2B7*2 and CYP2C8*4 alleles are responsible for diclofenac-induced liver injury.
4’-Hydroxy-diclofenac can form reactive benzoquinone imines that deplete hepatic glutathione and UGT2B7 catalyses the formation of another reactive metabolite: the diclofenac acyl glucuronide [34]. The penetration of diclofenac into human tissues has been reviewed. (Seefried et al. [35]) studied the penetration of diclofenac diethylamine into the synovial tissue and into the synovial fluid of knee with osteoarthritis following the topical application of diclofenac diethylamine gel 2.32% w/w 4 grams twice-daily. The concentration of diclofenac diethylamine in synovial tissue and in synovial fluid is about one-fourth and about one-half, respectively, of that in plasma indicating that, following topical application, diclofenac diethylamine penetrates into the synovial tissue and into synovial fluid in significant amounts. Miyatake et al. [36] studied the penetration of diclofenac sodium into human tissues following the application of two tapes containing 30 mg of diclofenac sodium and following the oral administration of a capsule containing 37.5 mg of diclofenac sodium. The concentration of diclofenac sodium in skeletal muscle is higher following the topical application and that in synovial membrane and in synovial fluid is lower following the topical application. As the concentration of diclofenac sodium in skeletal muscle is higher following the topical application the topical application is the preferred formulation of diclofenac sodium. (Brunner et al. [37]) measured the area under the concentration-time curve and the peak concentration of diclofenac in plasma, in subcutaneous muscle, and in skeletal muscle after repeated application of a spry gel containing 4% diclofenac and following the oral administration of enteric coated tablets containing 50 mg of diclofenac. The area under the concentration-time curve and the peak concentration of diclofenac in subcutaneous tissue and in the skeletal muscle are higher following the topical application suggesting that the topical application is the preferred formulation of diclofenac. Leuratti et al. [38] studied the pharmacokinetics of diclofenac sodium in healthy women and men following the administration of 75 mg of diclofenac sodium injected intravenously in 5, or in 15, or in 30 seconds. All pharmacokinetic parameters are similar according to the three injection times and the elimination half-life of diclofenac sodium is about 1.3 hours suggesting that diclofenac sodium is rapidly eliminated. These authors injected 75 mg of diclofenac sodium intravenously in 5 seconds, injected 75 mg of diclofenac sodium intramuscularly, and infused intravenously over 30 min 75 mg of diclofenac sodium. Except for the peak concentration, all pharmacokinetic parameters of diclofenac sodium are similar according the three formulations and the elimination half-life of diclofenac sodium is about 1.5 hours. The peak concentration of diclofenac sodium is higher following the intravenous bolus injection than following the intramuscular injection and the intravenous infusion suggesting that diclofenac sodium diffuses into the body more rapidly following the intravenous injection. The toxicity induced by diclofenac has been reviewed. Niu et al. [39] studied the toxicity caused by diclofenac in precision-cut jejunum slices obtained from human donors and added 600 and 400 μM of diclofenac in the incubation medium. Diclofenac depleted ATP, caused morphological damage, and leaked lactate dehydrogenase which are the toxic effects caused by diclofenac and the toxicity was greater following the inclusion in the incubation medium of 600 μM than 400 μM diclofenac. The addition of the cytochrome P-450 inhibitors ketoconazole or cimetidine in the incubation medium decreased the formation-rate of the 4’-hydroxy-diclofenac, reduced the consumption of diclofenac with consequent increase of toxicity. These results suggest that diclofenac can induce toxicity in humans. (Bort et al. [40]) observed that the acute toxicity caused by diclofenac in human hepatocytes is due to decrease of ATP concentrations and to impairment of ATP synthesis by mitochondria. The addition of cytochrome P-450 inhibitors proadifen or ketoconazole in the incubation medium decreases the consumption of diclofenac with consequent increases of hepatotoxicity. These results suggest that the toxic effect of diclofenac on human hepatocytes is caused by drug-induced decrease of ATP concentration and by impairment of ATP synthesis together with a futile consumption of NADPH. Tateishi et al. [41] stated that the hepatotoxicity induced by diclofenac is due to the formation of reactive quinone imine and acyl glucuronide metabolites that deplete hepatic glutathione. In conclusion, diclofenac, a phenylacetic acid derivative, is the most frequently used nonsteroidal anti-inflammatory drug in Europe. Diclofenac has analgesic, antipyretic, and anti-inflammatory activities and is used for long-term symptomatic treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, pain, primary dysmenorrhea, and acute migraine. The usual daily oral dose of diclofenac is 50 to 150 mg given in several divided doses. Formulations of diclofenac are available for oral and intravenous administration and topical application. Diclofenac produces adverse-effects (particularly gastrointestinal) in about 20% of patients. Diclofenac has been found efficacy and safe and the prophylaxis and treatment with diclofenac and the trials conducted with diclofenac have been reviewed. Diclofenac is hydroxylated into 4’-hydroxy- diclofenac by CYP2C9 and is glucoronated by UGT2B7. The penetration of diclofenac sodium into human tissues has been studied following topical application and oral administration and the concentration of diclofenac sodium in the skeletal muscle is higher following the topical application than oral administration. The pharmacokinetics of diclofenac sodium have been studied in healthy subjects following a single intravenous injection of 75 mg of diclofenac sodium and the elimination half-life of diclofenac sodium is about 1.3 hours. The toxicity caused by diclofenac has been studied in-vitro using human jejunum slices and human hepatocytes. In slices of human jejunum, the toxicity is caused by ATP depletion, morphological damage, and lactate dehydrogenase leakage and in human hepatocytes the toxicity is caused by a decrease in ATP concentration and impairment of ATP synthesis by mitochondria. In addition, reactive quinone imine and acyl glucuronide are responsible of the toxicity induced by diclofenac. The aim of this study is to review the clinical pharmacology of diclofenac.
In conclusion, diclofenac, a phenylacetic acid derivative
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals.
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.


