Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Shi-Feng Wang, Jin-Lin Mei, Le-Chen Shen, Qiu-Xia Xiao* and Liu-Lin Xiong*
Received: May 16, 2024; Published: June 03, 2024
*Corresponding author: Qiu-Xia Xiao, Department of Anesthesiology, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
Liu-Lin Xiong, Department of Anesthesiology, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
DOI: 10.26717/BJSTR.2024.56.008914
Background: The relationship between anesthesia and neural circuits has garnered increasing attention in
recent years.
Methods: This study conducted a comprehensive bibliometric analysis and review of research literature in
this field, focusing on publications, contributing countries, references, and keywords.
Results: Our analysis revealed a significant rise in publications from 2019 to 2021, indicating growing interest
and research activity in understanding the neural mechanisms underlying anesthesia. Among the contributing
countries, the United States and China emerged as the most prolific and influential, underscoring their leading
roles in advancing research in this area. Furthermore, we identified key authors who have made substantial
contributions to the literature, highlighting their impact on shaping the discourse surrounding anesthesia
and neural circuits. Keyword analysis uncovered several prominent themes, including general anesthesia,
consciousness, and specific anesthetic agents such as propofol, isoflurane, and ketamine. Additionally, our
study explored the potential relevance of anesthesia-induced effects on sleep, memory, and unconsciousness
to clinical practice. Through a review of relevant studies, we elucidated the specific pathways implicated
in these effects, shedding light on the underlying neural mechanisms. Moreover, our analysis delved into
the unique mechanisms of action of different anesthetic agents, such as sevoflurane, isoflurane, propofol,
ketamine, etomidate, and dexmedetomidine. We discussed their distinct impacts on neural circuits and their
implications for anesthesia-induced unconsciousness. Notably, while some agents like dexmedetomidine
primarily induced sedation, others like ketamine have potential applications beyond anesthesia, particularly
in the treatment of mood disorders.
Conclusions: Overall, our study underscored the importance of understanding the neural circuits involved in
anesthesia to enhance patient safety and optimize anesthesia practices. By elucidating the intricate interplay
between anesthetic agents and neural pathways, we can pave the way for the development of novel anesthetic
techniques and therapeutic interventions.
Keywords: Anesthetics; Neural Circuits; Brain; Memory; Bibliometric Analysis
List of Abbreviations: mPFC: Medial Prefrontal Cortex; PAG: Periaqueductal Gray Matter; WOS: Web of Science; EEG: Electroencephalogram; GABA: γ-Aminobutyric Acid; IPSC: Inhibitory Postsynaptic Potential; LOC: Loss of Consciousness; D1R: Dopamine D1 Receptor; DPM: Dorsal Paired Medial; VN: Vestibular Nucleus; MPTA: Midbrain Pontine Tegmental Area; CMT: Central Medial Thalamus; DMT: Dorsomedial Thalamus; PAC: Parietal Association Cortex; VLPO: Ventral Lateral Preoptic Area; PFC: Prefrontal Cortex; DMH: Dorsomedial Hypothalamic; MPB: Medial Parabrachial Nucleus; POA: Preoptic Area; NAc: Nucleus Accumbens; SCN: Suprachiasmatic Nucleus; LH: Lateral Hypothalamus; PVT: Paraventricular Nucleus of the Thalamus; BNST: Bed Nucleus of Stria Terminalis; VTA: Ventral Tegmental Area; TMN: Tuberomammillary Nucleus; aNGC: Anterior Nuclear Giant Cells; EAAT3: Excitatory Amino Acid Transporter Protein 3; AMPK: AMP-Dependent Protein Kinase; LTP: Long-Term Potentiation; LC: Locus Coeruleus; VLPO: Ventrolateral Preoptic Area
Approximately 250 million individuals worldwide undergo surgical anesthesia annually [1], a crucial practice aimed at alleviating pain, ensuring patient safety, and facilitating optimal surgical conditions during surgery or invasive procedures. Anesthesia refers to the administration of anesthetics to induce a regulated, temporary state of sensory loss or unconsciousness in individuals. It effectively addresses patients’ conditions through four aspects: analgesia, sedation, muscle relaxation, and loss of consciousness [2]. Prior studies indicate that general anesthesia does not globally suppress the brain as a whole; instead, it modulates the functioning of specific neural networks [3-5], particularly by regulating neural circuits. Neural circuits consist of intricate connections established among various types of neurons in the brain. The interplay between the electrophysiological characteristics of neurons in vivo and the organization of neural circuits gives rise to a sophisticated neuronal network [6]. Neural circuits exert their influence within organisms through various configurations such as series, parallel, feedforward, feedback, positive feedback, negative feedback, and more [7].
Research has demonstrated the association of neural circuits with the processes of pain generation and consciousness. For instance, Redinbaugh, et al. (2018) identified that thalamocortical circuits play a specific role in regulating the onset and cessation of consciousness in macaques [8]. Additionally, local circuits involving medial prefrontal cortex (mPFC)-midbrain periaqueductal gray matter (PAG) neurons have been implicated in the development of chronic pain [9]. The administration of anesthetics significantly mitigates the risk of pain by modulating these neural circuits. Understanding the current state of research, identifying research focal points, and forecasting future directions are essential for advancing knowledge in a particular field. Bibliometric analysis, employing mathematical, statistical, and quantitative methods, effectively examines the internal relationships and distribution patterns within literature [10]. Despite the increasing publication of relevant papers on nerve circuitry in anesthesia, there remains a dearth of systematic reports on measurement studies in this domain.
Hence, this study aims to address this gap by comprehensively analyzing all research papers on neural circuits and anesthesia indexed in the Web of Science (WOS). Our analysis encompasses publication trends, global contributions, keyword analysis, and more, aiming to delineate the predominant trends in the application of neural circuits in anesthesia research and provide guidance for future investigations. This paper employs bibliometric analysis to visually present four key aspects of anesthesia and neural circuits: publication trends, global contributions, reference mapping, and keyword co-occurrence. Additionally, it synthesizes information on neural circuits and their targets pivotal in the induction of general anesthesia by commonly used anesthetics. This synthesis serves to furnish a theoretical foundation for subsequent scholars interested in advancing research within this field.
The primary keywords utilized for the search were (“Anesthesia”) OR (“Anesthetics”) AND (“neuronal circuit” OR “neural circuit” OR “nerve circuits”). Relevant literature on anesthesia and neural circuits was collected from the Web of Science (WOS) database. The search methodology and screening process are outlined in Figure 1A. Our focus was on original articles, excluding other types such as commentaries and reviews. All data, presented in plain text format, encompassed information on countries/regions, titles, keywords, authors, institutions, and journals. The search was concluded on September 13th, 2022, to mitigate any discrepancies arising from database updates. For the analysis of country/region contributions and keyword significance, VOS viewer 1.6.18 was employed. In the network generated by VOS viewer, the size of each node indicated the number of publications, with larger nodes representing more publications. Links between nodes depicted the relevance among various parameters (countries/regions, institutions, authors, or keywords), with thicker links indicating stronger connections. Furthermore, domain overlay analysis was conducted using both VOS viewer 1.6.18 and Pajek 645.16. Data processing and visualization, including publication year statistics, citation frequency analysis, and the calculation of average citation time and H-index for each country, institution, or author per project, were carried out using Microsoft Excel 2019 and Origin. Given that our analysis was based on publicly available datasets, an ethical statement was deemed unnecessary for this study.
Results Publication Trend
A total of 164 articles concerning the utilization of neural circuits in anesthesia were incorporated into the final bibliometric analysis. Examination of annual publication figures revealed a notable upward trajectory in research output over the years, particularly evident from 2010 to 2017 (with 5-15 papers annually) and 2018 to 2022 (with 10-25 articles annually). A significant surge in publications from 2019 to 2021 underscores the growing popularity and widespread attention garnered by the application of anesthesia in neural circuits (Figure 1B).
Global Contribution
Regional co-authorship analysis revealed interconnectedness among 18 countries and regions, with the USA serving as a research hub, maintaining close collaborations with China, Germany, and the UK (Figure 2A). Despite relatively lower research outputs from Japan, Italy, and Israel, the intensity and volume of their contributions suggest strong interrelations with other countries and significant influence on international research. To visualize the global distribution of publications by country, VOS viewer was utilized and normalized using the correlation intensity method, with a minimum publication threshold set at two. Approximately 19 countries, including the United States (USA), China, Canada, the United Kingdom (UK), and others, met this threshold (Figure 2B). In terms of publication numbers, the USA emerged as the most prolific contributor with 85 publications, followed by China (39), the UK (14), and Japan (12) (Table 1). Notable journals such as Science, Current Biology, Proceedings of the National Academy of Sciences, and Nature Reviews Neurology played a significant role in disseminating research on anesthesia and neural circuits (Figures 2C & 2D). Regarding total citations, the top three countries were the US (3181), China (551), and the UK (486). The highest average citation rates were recorded for Switzerland (96.20), Canada (92.88), and Israel (56.14) (Table 1). These findings underscore the predominant influence of the USA and China in this field.
Authors Distribution
The three most prolific authors in terms of articles published from 1997 to 2022 were Brown Emeryn (12 publications), Akeju Oluwaseun (8 publications), and Purdon Patrick (7 publications), all based in the United States. Following them was Hai-Long Dong (5 publications) from China (Table 2). When considering citations, the top three authors were Brown Emeryn (1126 citations), Purdon Patrick (937 citations), and Pavone Kara (562 citations), also all from the United States (Table 2).
Journal Distribution
An overlay analysis of the cited journals reveals that ‘Anesthesiology’ is the journal with the highest number of citations, followed by ‘Frontiers in Neural Circuits’ (Figure 2C). Notably, in recent years, journals like ‘Current Neuropharmacology’ and ‘Frontiers in Neuroscience’ have experienced a rise in citations (Figure 2D).
Table 1: The Top 11 countries contributing to publications related to neural circuits for anesthesia or anesthetics.
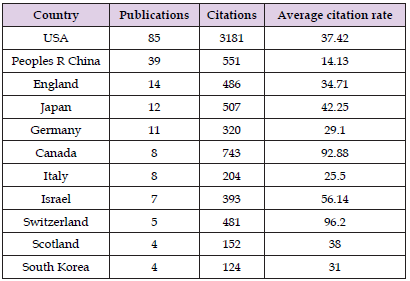
Table 2: The top authors in the field of Neural Circuits for anesthesia or anesthetics ranked by publication and citation numbers.

Reference Mapping
We compiled the 25 most cited references (Figure 3B), with the most cited being Franks N, 2008, published in Nature Reviews Neuroscience, volume 9, issue 5, pages 370-386 (doi: 10.1038/nrn2372).
Keyword Copolymerization Analysis
Keywords serve as a succinct representation of the core themes within a paper. Conducting a co-occurrence analysis aids in systematically comprehending the research hotspots, progress, and internal relationships within the domain of anesthesia-related neural circuits. In our study, we set the threshold for keyword occurrences in titles at a minimum of 5. Using VOS viewer for this analysis, we established a threshold of at least 16 occurrences for a keyword to be deemed significant. As a result, 234 keywords were identified and grouped into six clusters: general anesthesia, consciousness, propofol, isoflurane, ketamine, and brain (Figures 4A & 4B). The temporal distribution of these keywords was visualized based on the average year of publication, demonstrating ongoing updates and advancements in research pertaining to anesthesia or anesthetic-related neural circuits. Recently emerging keywords such as ‘plasticity’, ‘bispectral index’, and ‘propofol’ underscore growing research interests in these areas (Figure 4C). A co-polymerization analysis, conducted using Cite Space, revealed a concentration of studies on keywords including ‘mechanism’, ‘isoflurane’, ‘synaptic plasticity’, and ‘volatile anesthetic’ from 1994 to 2022 (Figure 4D). Over the last three years, there has been a consistent trend in the number of citations across all keywords. Clustering analysis along the time axis suggests that ‘neural circuits’, ‘mechanisms’, ‘isoflurane’, ‘synaptic plasticity’, and ‘volatile anesthesia’ have been focal points of recent research, potentially indicating directions for future investigations (Figure 4E).
Overview of Research on the Neural Circuit in General Anesthesia
This paper presents a comprehensive bibliometric analysis of research on anesthesia and neural circuits, focusing on publications, contributing countries, references, and keywords. The analysis reveals a steady increase in publications from 2019 to 2021, reflecting growing interest and attention in this field. Notably, nineteen countries and regions have contributed papers on anesthesia and associated neural circuits, with China and the USA emerging as the most prolific and influential contributors. Among individual authors, the top three, all from the US, include Brown Emeryn (12 publications), Akeju Oluwaseun (8), and Purdon Patrick (7), followed by Hai-Long Dong (5) from China. In terms of citations, the leading authors are also from the USA, with Brown Emeryn (1126 citations), Purdon Patrick (937), and Pavone Kara (562) garnering the highest citation counts. This underscores the significant role that the USA plays in research related to anesthesia and neural circuits. Keyword analysis reveals current focus areas in this field, including general anesthesia, consciousness, propofol, isoflurane, ketamine, and brain studies. The keyword timeline clusters suggest that neural circuits, mechanisms, isoflurane, synaptic plasticity, and volatile anesthesia are prominent research hotspots in recent years and likely directions for future research.
Concept and Function of General Anesthetics
General anesthetics, administered via inhalation, intravenous injection, or intramuscular injection, exert a reversible depressant effect on the central nervous system. Clinically, they are categorized into two main types: intravenous and inhalation anesthetics [11]. These agents vary in potency and efficacy for different neurobiological effects (Table 3). They possess sedative and analgesic properties; for instance, isoflurane and propofol are commonly utilized for sedation [12,13]. Moreover, certain anesthetics have therapeutic applications for specific conditions; for example, isoflurane can be employed in the treatment of epilepsy [14], while propofol has demonstrated effectiveness in alleviating depression [15]. Nitrous oxide and ketamine induce analgesia by inhibiting glutamate and nicotinic receptors while activating potassium channels, such as TREK-1. Dexmedetomidine mediates analgesia through the locus coeruleus-norepinephrinergic circuit [16]. Each anesthetic influences unique oscillatory dynamics, potentially associated with the modulation of neural circuits downstream of the brainstem and hypothalamus [17], and alters electroencephalogram (EEG) wave patterns and frequencies. Notably, Oluwaseun Akeju’s study administering dexmedetomidine and propofol to volunteers revealed a deeper unconsciousness induced by prolonged large-amplitude slow oscillations [18].
Ketamine, for instance, reduces γ-aminobutyric acid (GABA) inhibitory postsynaptic potential (IPSC)-induced β/γ (13-40 Hz) brain wave oscillations [19]. The actions of anesthetics involve multiple neural groups; for instance, propofol and sevoflurane inhibit neuronal activity and/or increase inhibitory synaptic connections in thalamocortical circuits [20] and induce loss of consciousness (LOC) by activating dopamine D1 receptor (D1R)-expressing neurons in the nucleus ambiguous [21]. The role of anesthesia in memory formation is significant. Research by Nir Samuel, et al. revealed that circuits within the macaque’s amygdala and dorsal anterior cingulate cortex maintain aversive memory acquisition under ketamine and midazolam sedation [22]. In Drosophila, pentazocine released from dorsal paired medial neurons (DPM) inhibits memory formation during isoflurane anesthesia [23]. Improper use of anesthetics can lead to adverse effects; for instance, etomidate increases susceptibility to pneumonia in trauma patients [24], and propofol can cause propofol infusion syndrome (PRIS), potentially resulting in refractory bradycardia and cardiac arrest [14]. In surgical applications, however, anesthetics significantly reduce risks and enhance safety.
Study on Neural Circuit Involved in Different Anesthetics
Based on the insights derived from the aforementioned analyses, we conducted a comprehensive review of studies focusing on anesthesia and neural circuits, with particular emphasis on areas such as sleep, memory, or unconsciousness (Table 4, Figures 5 & 6). We delve into their potential relevance to clinical anesthesia and elucidate the specific pathways implicated in their effects, such as the inhibition of NMDA receptor activity and the upregulation of GABA channel opening (Figure 7). Therefore, drawing on the findings of these analyses, we conducted a review of studies on anesthesia and neural circuits, particularly those related to sleep, memory, or unconsciousness (Table 4, Figures 5 & 6). We explore their potential relevance to clinical anesthesia and the specific pathways implicated in their effects, such as the inhibition of NMDA receptor activity and the upregulation of GABA channel opening (Figure 7).
Sevoflurane: Sevoflurane, a well-tolerated volatile anesthetic commonly utilized for inhalation induction, is particularly suitable for pediatric patients. Its clinical effects may include bradycardia, hypotension, cough, and vomiting. Sevoflurane enhances the activity of GABAA-type and glycine receptors [25] and activates double-pore potassium channels [26-30], influencing EEG wave patterns. Notably, sevoflurane induces a significant increase in EEG power in children from infancy to 6 years of age, stabilizing at around 21 years. α wave (8-13 Hz) coherence is a prominent EEG feature associated with sevoflurane-induced unconsciousness in adults [31]. Multiple nuclei groups are implicated in sevoflurane anesthesia. The vestibular nucleus (VN), midbrain pontine tegmental area (MPTA), central medial thalamus (CMT), dorsomedial thalamus (DMT), and parietal association cortex (PAC) within the thalamocortical circuit have been identified as mediators of sevoflurane anesthesia [32]. Additionally, neural circuits regulating consciousness under sevoflurane anesthesia enhance endogenous sleep network activity by activating the ventral lateral preoptic area (VLPO) nucleus in the hypothalamus [33,34]. The induction of prefrontal cortex (PFC)-dorsomedial hypothalamic nucleus (DMH) glutamatergic and GABAergic-VLPO circuits in the DMH boosts neuronal activity [35]. The inhibition of postsynaptic GABAA receptors and background potassium channels in the medial parabrachial nucleus (MPB) facilitates anesthesia induction [36], while the inhibition of presynaptic Ca2+ channels in hippocampal interneurons and the reduced firing frequency of CMT neurons suppress emergence during anesthesia [37].
Emergence from sevoflurane anesthesia involves various circuits and nuclei. Research by Sarah L. Reitz, et al. found that activation of preprotachykinin-1 (Tac-1) neurons in the preoptic area (POA) induced emergence in mice [38]. Similarly, neurons with dopamine receptors in the nucleus accumbens (NAc) are involved in consciousness regulation under sevoflurane anesthesia and promote emergence [21]. Blue light stimulation enhances neuronal activity in the suprachiasmatic nucleus (SCN) under sevoflurane, inducing high levels of c-Fos expression in the prefrontal cortex (PFC) and lateral hypothalamus (LH), thereby facilitating emergence [39]. Transient stimulation of the paraventricular nucleus of the thalamus (PVT)-bed nucleus of stria terminalis (BNST) pathway also induces behavioral emergence in mice and reduces anesthesia depth during induced burst suppression [40]. It has been observed that sevoflurane not only induces anesthesia but also enhances the intrinsic excitability of cerebellar granule cells, shaping neuronal communication without altering the neural circuit [41].
Isoflurane: Isoflurane, a halogen-class inhalation anesthetic, was introduced clinically before sevoflurane and is predominantly used for animal anesthesia. Its molecular formula is C3H2ClF5O. Isoflurane targets two-pore potassium channels, NMDA receptors, glycine receptors, and GABAA receptors. Its interaction with GABAA receptors, particularly within thalamocortical circuits, modifies inhibitory postsynaptic current production in excitatory spiny stellate cells and cone cells of the auditory cortex [42]. Similarly to sevoflurane, isoflurane prevents action potential inhibition by activating potassium channels in the CMT and reducing central thalamic neuronal firing [37]. Isoflurane’s effects extend to the modulation of GABAergic neurons in the lateral septum, which send inhibitory signals to the ventral tegmental area (VTA), influencing the emergence and depth of anesthesia [43,44]. It directly activates neurons in the VLPO [45] and alters the activity of multicellular biological interneurons [46].
Activation of the histaminergic tuberomammillary nucleus (TMN) induces emergence from isoflurane anesthesia. In Drosophila, TMN damage following isoflurane exposure leads to a loss of the righting reflex and extended emergence time, although it does not significantly affect sensitivity to other anesthetics like propofol, pentobarbital, and ketamine [47]. Post-isoflurane anesthesia, stimulation of anterior nuclear giant cells (aNGC) robustly activates specific neuronal groups in the reticular activating system, enabling extensive reactivation of cortical function and motor behavior in rodents. This involves coordinating multiple emergence-promoting circuits, such as the locus coeruleus and parabrachial nucleus [48]. Mice lacking excitatory amino acid transporter protein 3 (EAAT3) show increased sensitivity to isoflurane-induced anesthesia, mediated by the hypothalamic sleep neural circuit [49]. Light stimulation activates orexinergic neurons in the BF-LC pathway, promoting emergence and motor recovery in rats [50,51]. Research suggests that isoflurane anesthesia primarily affects cortical rather than subcortical structures in mice, leading to diminished thalamus-cortex connections [32]. Connectivity between the solitary bundle nucleus and the ventral lateral or suspected nucleus at the head of the medulla oblongata also decreases under isoflurane anesthesia [52]. Isoflurane influences memory by inhibiting interneurons within the cortical hippocampal circuit [53]. It also impacts neurons in the anterior border of the medial prefrontal limbic and inferior limbic areas, affecting associative learning processes [54]. Additionally, isoflurane affects the activity of cortical limbic and reward-related areas, such as the basal nucleus and limbic thalamic nuclei, which play roles in sensory association processes, emotions, and learning and memory-related behaviors [55].
Ketamine: Ketamine, a general anesthetic administered intravenously, is chemically related to phencyclidine and has the formula C13H16ClNO HCl. It antagonizes NMDA receptors and non-competitively inhibits norepinephrine transporter proteins. Additionally, ketamine upregulates AMPA receptors and activates μ opioid receptors, providing anesthetic, analgesic, amnesic, and sedative effects [56]. It also inhibits Na+ and Ca2+ channels and interacts with monoaminergic, cholinergic, muscarinic, and nicotinic receptors [57]. Distinct from other anesthetics, ketamine selectively inhibits the medial thalamic nucleus, blocks reticular tract conduction in the spinal cord, and activates the limbic system, thereby inducing “dissociative anesthesia”, which is independent of its analgesic properties [58-60].
Ketamine’s blockage of NMDA receptors in cortical interneurons is more pronounced than in pyramidal neurons, resulting in the relative inhibition of the former and hyperactivity in glutamate-mediated pyramidal neurons. This leads to rigidity, shallow sedation, amnesia, and significant analgesia [61,62]. At higher doses, ketamine induces unconsciousness typical of general anesthesia [63]. First introduced in the 1960s as an anesthetic [64], ketamine has potential for psychiatric dependence and is unique among intravenous anesthetics for its analgesic properties. Its clinical use is sometimes limited due to side effects like rapid heart rate, hypertension, and hallucinations [65]. However, its lower respiratory depression capability makes it a preferred choice in settings without reliable ventilatory support. Ketamine has been explored for treating mood disorders [66], addiction [67], schizophrenia [68], and for pain relief [69,70].
Under ketamine anesthesia, EEG characteristically shows a γ burst [71,72], a decrease in α power, and an increase in γ power. This γ power amplification is pronounced in anesthetized rats and monkeys [73]. Ketamine affects the thalamocortical network by altering sensorimotor rhythm amplitudes and interregional blood flow but does not inhibit the thalamus directly [74.,75]. It activates various emergence centers, such as the mesencephalon and brainstem [76], instead of the ventral lateral preoptic nucleus [77]. Ketamine enhances connections between the prefrontal cortex and hippocampus, remodels cortico-marginal-striatal circuits, and alters overall brain functional connectivity [66]. It consistently elevates activity in the medial substantia nigra, ventral tegmental area, frontal cortex, and ventral striatum, potentially improving depression scores in major depressive disorder [78,79] patients through its rapid and sustained effects on the midbrain’s limbic neural network. During ketamine administration, feedback connections in the frontoparietal network progressively weaken and become significantly inhibited post-loss of consciousness [72,80]. Anesthesia studies in monkeys have revealed changes in brain activity regionally, with increases in the fractional amplitude of low and medium frequency fluctuations in various brain regions [81]. Resting-state functional MRI confirms acute changes in brain circuit intensity and connectivity under ketamine, notably disrupting the functional connectivity of the default mode, cognitive control, and medullary affective networks. Currently, conventional treatments for major depressive disorder are slow-acting and less effective with a higher recurrence rate [82,83]. Recently, ketamine has garnered attention as a rapid-acting antidepressant but remains underexplored in anesthesia-induced loss of consciousness studies.
Propofol: Propofol, chemically known as 2,6-diisopropylphenol with the molecular formula C12H18O, is a widely used and extensively studied short-acting intravenous anesthetic in clinical practice. It primarily suppresses neuronal activity by enhancing GABAA receptor-mediated inhibition in the cortex, thalamus, and brainstem [27,84-86]. Propofol facilitates the opening of chloride channels, increasing inward chloride flow, and consequently hyperpolarizing postsynaptic membranes [87]. It is commonly used for the induction and maintenance of sedation and general anesthesia, especially in outpatient procedures. However, its administration can lead to side effects such as respiratory depression, apnea, hypotension, injection pain, and allergies. Dosage of propofol is critical [88], a lower dosage may cause excitation instead of sedation, linked to the activation of AMP-dependent protein kinase (AMPK) [89] in neurons and LTS cell-mediated excitation of cortical interneurons [90-92]. Conversely, a higher dosage can result in unconsciousness [93,94]. The EEG patterns under propofol anesthesia are characterized by frontal alpha oscillations (8 to 12 Hz), delta oscillations (1 to 4 Hz), and slow oscillations (0.1 to 1 Hz). Large amplitude slow oscillations, indicating prolonged neuronal silence, are induced to deepen unconsciousness [18]. α oscillations, synchronized between the thalamus and medial prefrontal cortex, can trigger coherent thalamocortical δ oscillations, which are critical for consciousness.
The transition from sedation to unconsciousness might be attributed to alterations in the GABAergic network across the cortex, thalamus, and brainstem, resulting in an inhibited brain state [93,94]. However, research by Li Ma, et al. indicates that this inhibition is not uniform across the brain. Disproportionate inhibition of functional connectivity in frontal and parietal regions can induce coherent oscillations and intensify certain frontoparietal interactions in the motoneuron [95]. Similarly, Laura D Lewis, et al.’s intracranial cortical EEG recordings during propofol anesthesia revealed uneven local cortical dynamics, suggesting that some cortical areas under anesthesia might be in a state of emergent inhibition while adjacent areas exhibit continuous activity [96]. This uneven activity is evident in the nucleus accumbens during anesthesia, where a significant increase in c-Fos expression in VLPO neurons is observed, leading to loss of consciousness [76]. Stimulating the nucleus ambiguus (NAc) can prolong propofol-induced loss of consciousness by inhibiting functional connectivity between NAc-dorsal raphe (DR) and NAc-cingulate cortex (CG) [97]. Additionally, propofol reduces the excitability of cholinergic neurons in the mouse basal forebrain via GABAA receptors, affecting the basal forebrain’s critical role in sleep-wake regulation [98]. In terms of memory behavior effects, propofol’s sustained activation of GABAA-type receptors leads to long-term potentiation (LTP) injury in the CA1 region of the hippocampus, reducing cortical neuron density [99]. This decrease in excitatory input to cortical pyramidal neurons, along with reduced activity of pyramidal and cortical inhibitory interneurons, has been linked to impaired cognitive and behavioral cortical circuits in neonatal mice, resulting in cognitive and behavioral deficits [100].
Etomidate: Etomidate is a non-barbiturate intravenous anesthetic, primarily mediating anesthesia by enhancing GABAA receptors [101]. It acts rapidly and minimally impacts the respiratory and cardiovascular systems, making it particularly suitable for geriatric patients with cardiovascular and cerebrovascular diseases and an ideal induction agent for cardiac anesthesia [102]. However, etomidate’s inhibition of adrenal cortical endosteroid synthesis has sparked considerable controversy. There is currently no consensus on whether its use should be limited to specific scenarios, especially in cases of infectious shock and critical care patients [103-105]. In a study by Zhang L, et al., EEG data from 20 patients who underwent etomidate- induced general anesthesia were analyzed. The findings suggested that the neural circuit mechanisms inducing unconsciousness were associated with evoked δ-, θ-, α-, and β-wave oscillations, along with enhanced δ-wave coherence [106]. At present, detailed information on the neural circuits involved in etomidate anesthesia remains temporarily unavailable.
Dexmedetomidine: Dexmedetomidine, an agonist of central and peripheral α2-adrenergic receptors (α2-AdRs), induces hyperpolarization, decreases norepinephrine release, and produces sedative, anxiolytic, and analgesic effects. During sedation, spindle oscillations (12 to 16 Hz) in the EEG pattern are significantly induced, akin to those observed during general anesthesia with propofol18. Dexmedetomidine acts on brainstem sleep circuits, binding to presynaptic α2-adrenergic receptors in the locus coeruleus (LC) [107] and mediating sedation that mimics NREM sleep [107,108]. Nelson LE, et al. demonstrated that dexmedetomidine-induced sedation could be reversed by impairing the LC or by injecting an α2-AdR inhibitor into the LC of rats [108]. They observed that activating α2-AdR in nodal papillae nuclei to inhibit the ventrolateral preoptic area (VLPO) subsequently inhibited the tuberomammillary nucleus (TMN) via GABA release, similar to selective injections of anesthetics or GABA agonists into the nucleus. However, dexmedetomidine does not significantly alter cortical EEG frequencies, and subcortical function remains connected in anesthetized mice, maintaining interaction between the cortex and thalamus [32,109]. Notably, dexmedetomidine does not reliably induce unconsciousness; its primary use is in sedation, particularly beneficial in “awake craniotomy” where patient communication is necessary [108]. It synergistically enhances the effects of other sedative and analgesic drugs, thus reducing their required dosages. Its clinical application extends to ICU sedation and as a supplement in intraoperative general anesthesia. Our findings are summarized below. Firstly, the presence of neural connections does not always imply functionality.
The existence of structural connections does not guarantee functional connectivity, which necessitates further exploration. Secondly, the current understanding of neural circuits in anesthesia is largely based on animal experiments, suggesting variability in neural circuits between humans and animals due to differences in nervous system development. Therefore, more reliable experimental models are needed for validation. Thirdly, variations in brain structure, synaptogenesis, neuronal differentiation, neurochemical signaling, and myelination at different stages of cerebral development indicate that adult neural network systems cannot be directly applied to children’s brains of various ages [110,111]. Fourthly, no single brain region or mechanism has been definitively linked to consciousness, indicating that consciousness arises from complex functional interactions across different neural circuits and neurons in varying temporal and spatial contexts. Identifying where and when anesthesia targets or takes effect remains a challenging endeavor. It is also difficult to discern common mechanisms among the diverse changes in unconsciousness induced by different anesthetics [112].
In conclusion, our bibliometric analysis of the literature on anesthesia and neural circuits reveals a growing interest in understanding the intricate relationship between anesthetic agents and neural circuitry. Specifically, there is significant research focusing on the involvement of cortex-cortex and thalamus-cortex pathways with anesthetics such as sevoflurane, isoflurane, and propofol. Dexmedetomidine, primarily used for sedation, has been investigated for its effects on brainstem sleep circuits but does not reliably induce unconsciousness. Ketamine, while garnering attention for its potential as a rapid-acting antidepressant, has limited research on its neural circuitry during anesthesia. Studies on the neural circuits related to etomidate are scarce and require further investigation. Overall, our analysis highlights a notable gap in research regarding the precise neural circuits involved in the mechanisms of general anesthesia. Further exploration in this area is crucial for advancing our understanding of anesthesia-induced unconsciousness and developing safer and more effective anesthetic agents and techniques.
Not applicable.
Not applicable.
All information and images contained in this article obtained informed consent from all authors.
Not applicable.
The authors declare that they have no competing interests.
Shi-Feng Wang collected the data and wrote the article, Jin-lin Mei and Le-Chen Shen completed the picture production, Qiu-Xia Xiao participated in manuscript revision, and Liu-lin Xiong reviewed the full text.
None.
No.


