Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Oluwafemi Royal Aliu
Received: April 24, 2024; Published: May 06, 2024
*Corresponding author: Oluwafemi Royal Aliu, University of Reading, United Kingdom, MyFarmbase Africa, Nigeria
DOI: 10.26717/BJSTR.2024.56.008858
Effect of land use on manganese distribution in soil has been carried out in countries like Iran and Lagos city of Nigeria, however, there is still a great scarcity of information regarding the concentration of manganese in the ancient cities of Nigeria (Abeokuta and Ibadan) based on the different forms of land use. The study aims to evaluate the impact of land use on manganese distribution in these soils under 8 different land uses (crop farm, animal farm, education, residential, roadside, and industrial, market and forest). Soil samples were collected at two different depths (0-20cm. 20-40cm with 3 replicates for each land use. Downward mobility of manganese was evaluated up to the depth of 40cm. The range of the manganese concentration in the soils of Abeokuta was 0.05-330 mg/ kg, while that of Ibadan was 0.36-560 mg/ kg. The results showed that the levels of manganese concentration in the various soils under the different land uses were found to be at tolerable levels compared to the world median of allowable levels of nickel concentration in natural soil. Correlation between manganese and other soil parameters including other heavy metals (Cu, Zn, Cr, Cd, Ni, Pb), soil pH, electrical conductivity, soil particle size, total nitrogen, phosphorus and carbon showed a positive correlation between the soil parameters except for soil pH, silt, and clay which are negatively correlated with soil manganese. The analysed soils were free from contamination but showed high concentrations in some soils.
Keywords: Land Use; Manganese; Distribution; Soils
Abbreviations: CEA: Culture and Education Area; CG: Classical Gardens; PGS: Public Green Space; RA: Residential Area: RSA: Roadside Area; IA: Industrial Area; Pb: Lead; Cd : Cadmium; Cu: Copper; Ni: Nickel; Zn: Zinc; Mn: Manganese; Co: Cobalt; Al: Aluminium; Fe: Iron; OM: Organic Matter; BCFs: Bio-Concentration Factors; MMED: Mass Median Equivalent Diameter; GPS: Global Positioning System; AAS: Atomic Absorption Spectrophotometry
Any toxic metal may be called heavy metals, irrespective of their atomic mass or density (Neetu, et al. 2011). Such heavy metals as mercury, arsenic, copper, cyanide, iron, lead, cadmium, chromium, nickel, and manganese are found in highly urbanized areas (Louella et al. 2006). It has been proven that Manganese is a heavy metal and it is very dangerous to the health and to the environment. It may also be known as a hazardous metal as high concentrations may harm an organism and accumulate in animal bodies to produce a variety of diseases.
High levels of heavy metal toxicity are being found in urban soils (Adeniran, Ilugbami & Oyeniran, [1]). Research has indicated that soils impacted by the oxidation of pyritic minerals exhibit elevated levels of metal contamination. Wildlife can become poisonous due to heavy metals. Urban soils have been significantly damaged due to intense human activity and high population density. As a result, a multitude of environmental concerns have surfaced, with heavy metal contamination continuing to be a primary concern [1]. Numerous sources, including automobile emissions, the chemical industry, the burning of coal, the deposition of dust and other suspended materials in the environment, and municipal solid waste, can discharge pollutants into the atmosphere. Heavy metals have been steadily introduced to urban soils by these pollutants, and even after the sources of pollution have been eliminated, these metals will still be there for many years. A hazardous disease called manganese poisoning is brought on by prolonged exposure to manganese [2].
Manganese-induced symptoms are often caused by exposure to ambient manganese air concentrations more than 5 mg Mn/m3. It may cause low energy, harm to the blood’s essential organs the liver, kidneys, lungs, and central nervous system—as well as impaired or diminished brain and central nervous system function. Long-term exposure can induce a degenerative process of the muscles and nervous system that resembles multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, and dystrophy [3,4]. Allergies are rare, and persistent long-term contact with certain metals (or their compounds) can potentially lead to cancer. Manganese, though widely distributed in soils, also occurs naturally in food and water and unlike some heavy metals with no known biological function, manganese is an essential nutrient and in trace amounts is very essential for growth and development. Land use affects and modifies soils positively or negatively as the case may be. Soil contamination is growing alarmingly and it is grossly determined by land use (Mercy et. al, 2013). The use of land cannot be over-emphasised in every sphere of human activities. The land encompasses all the features of natural resources including soil [5]. The positive results from the use of land are appreciated since they improve the standard of living of human beings either directly or indirectly. However, the adverse effects which sometimes result from the different land use can be detrimental to human beings directly and indirectly. There hasn’t been much study done on how heavy metals are distributed across various land uses, though. It is desirable to do research on the amounts of heavy metals in various urban soil types because the contaminants in these soil types may have distinct effects on public health [6,7].
This study has been carried out in countries like Iran by Sayadii et al. (2013), also in Spain, by Eduardo et al. (2008) and Nigeria by Elias et al. (2008) and in actual sense, the concentration of manganese in the ancient cities of Nigeria (Abeokuta and Ibadan) has yet been unknown based on the different forms of land use [8]. This study is therefore aimed at studying the impact/effect of various land uses, including business area (BA), classical gardens (CG), culture and education area (CEA), public green space (PGS), residential area (RA) and roadside area (RSA), industrial area (IA) on the distribution of Manganese in Abeokuta and Ibadan soils as it will provide us with scientific data for evaluation of contamination level in the soils. The objectives of this study are to evaluate the effect of various land uses on the distribution of manganese in some soils of Abeokuta and Ibadan and to evaluate the downward mobility of manganese in some soils of Abeokuta and Ibadan [9].
Although heavy metals are naturally occurring components of the earth’s crust, human activity has significantly changed the geochemical cycles and biological balance of these elements. Because of this, metals accumulate in plant portions that include secondary metabolites, which give rise to a specific pharmacological action. Human health risks can arise from prolonged exposure to heavy metals as manganese, cadmium, copper, lead, nickel, and zinc [10]. As well as being an ill-defined subset of elements with metallic characteristics, heavy metals are also hazardous metals. These consist of actinides, lanthanides, transition metals, and some metalloids [11]. According to one source, heavy metals include common transition metals like copper, lead, and zinc. These metals are responsible for contamination of the environment from sources including leaded gasoline, industrial effluent, and acid rain’s leaching of metal ions from the soil into lakes and rivers. Any species of metal (or metalloid) that appears in an unwelcome location or in a concentration or form that has negative effects on people or the environment might be classified as a “contaminant.” Lead (Pb), cadmium (Cd), copper (Cu), nickel (Ni), and zinc (Zn) are examples of metals and metalloids. Other less frequent metallic pollutants are molybdenum (Mo), manganese (Mn), cobalt (Co), and aluminium (Al).
Heavy Metal and Living Organism
Heavy metal requirements differ among living things. Humans need iron, cobalt, copper, and manganese. At greater amounts, all metals are hazardous. The organism may be harmed by excessive concentrations. Some heavy metals, like lead and mercury, are poisonous and have no known helpful or essential effects on living things. Over time, they may accumulate in the bodies of animals and cause catastrophic sickness [12].
Types of Heavy Metals and their Effect on Human Health
There are two ways that heavy metals impair metabolic processes: 1) They build up and cause malfunctions in important glands and organs including the heart, brain, etc.
2) They interfere with the biological function of the essential dietary minerals by moving them from their natural location. However, living in a heavy metal-free environment is not feasible [13].
Heavy Metals and Environmental Pollution
The content of metals in soil can vary from less than one to up to 100,000 mg/kg. The primary class of inorganic pollutants are heavy metals, which are found in a sizable portion of the land due to their usage in municipal compost or sewage, pesticides, fertilizers, and leftovers from metalliferous mining and smelting enterprises. Regardless of the source of the metals in the soil, high concentrations of various metals can lead to reduced crop yields, poor quality agricultural output, and deteriorating soil quality, all of which pose serious risks to the health of people, animals, and ecosystems [14,15].
Micronutrients (Fe, Mn, Zn and Cu)
Certain elements that plants need in extremely tiny amounts are referred to as micronutrients. This phrase is typically used to describe elements found in plant tissues at concentrations of less than 100 mg/ kg (Foth and Ellis, 1997). The same authors state that, in contrast to boron and molybdenum, the four vital micronutrients that are present in soil as cat ions are zinc (Zn), copper (Cu), iron (Fe), and manganese (Mn). An important process for eliminating micronutrients from the soil solution is adsorption of micronutrients, either by soil OM or by clay-size inorganic soil components (Foth & Ellis, 1997). As a result, each can be provided as a soluble salt, by mineral weathering, or by mineralizing organic matter. This increases the soil’s supply of soluble micronutrients (Foth & Ellis, 1997). Parent material, soil response, soil texture, and soil organic matter are factors that impact the availability of micronutrients (Brady and Weil, 2002). According to Tisdale et al. (1995), micronutrients show negative relationships with coarser sand particles and favourable relationships with fine mineral fractions like silt and clay. This is because these elements diffuse due to their high moisture retention (Tisdale et al., 1995). Micronutrient availability is also highly influenced by the amount of organic matter in the soil. As per Hodgson’s (1963) findings, the existence of organic matter (OM) might enhance the accessibility of certain elements by providing soluble complex agents that disrupt their fixation. The primary chemical factors influencing sedimentary manganese cycling are the oxygen content of the surrounding water, the oxygen’s capacity to penetrate the sediments, and the presence of benthic organic carbon [16,17].
Manganese in soil has the ability to leach soluble substances from it as well as disperse into the air and water as particulate particles. The two primary variables that impact the solubility of manganese in soils are the oxidation-reduction potential and pH. Aquatic biota has the ability to significantly bio-concentrate manganese in water at lower tropical levels. Bio-concentration factors (BCFs) have been estimated for the following: fish (35-930), phytoplankton (2500-6300), marine microalgae (300-5500), intertidal mussels (830-830), and freshwater and marine plants (2000-20,000). While dissolved oxygen has little effect, temperature and pH have a considerable impact on the absorption of manganese by fish and other aquatic invertebrates [18- 20]. It has been shown that manganese absorption rises when salinity falls. The concentrations of manganese in the air are typically highest in urban areas (65-166ng/m3), greater in rural regions (40ng/m3), and lowest in distant places (0.5-14ng/m3). Quantities of manganese in the air can exceed 8000ng/m3, with source-dominated locations often having the greatest quantities. Air near foundries may have annual averages of 200-300ng/m3 of manganese, whereas air near ferro and silico-manganese industries may have concentrations of exceeding 500 ng/m3. In naturally occurring waters with minimal human interference, the levels of dissolved manganese can vary between 10 and more than 10,000μg/litre. Nonetheless, naturally occurring surface waters typically have dissolved manganese concentrations of less than 200μg/litre and very seldom surpass 1000μg/litre. Manganese is an important mineral for bacteria, plants, and animals. Manganese concentrations in river sediments ranged from 410 to 6700 mg/ kg dry weight; silt from an urban lake getting inputs from industrial sources. Terrestrial plants need between 10 and 50 milligrams of manganese per kilogram of tissue. The critical nutritional values of different species and cultivars of the same species differ significantly [21-23]. Manganese-deficient plants grow in calcareous soils, particularly those with high levels of organic matter and poor drainage.
Environmental Transport, Distribution, Transformation, and Accumulation Transport and Distribution Between Media
Although elemental manganese and inorganic manganese compounds have very low vapour pressures, they can nevertheless be found in the atmosphere as suspended particulate matter from soil erosion or industrial emissions (US EPA, 1984). Manganese is likely to be found in the troposphere as mineral complexes linked to its natural occurrence in soil or rock, or in oxide, sulphate, or nitrate forms (Stokes et al., 1988). Particles containing manganese are composed of manganese and its compounds, which are mostly extracted from the atmosphere by rain or gravity settling (US EPA, 1984). Manganese-containing soil particle matter can travel through the atmosphere. The size, density, wind direction, and speed all have a major role in determining the destiny and movement of manganese in the atmosphere. It is estimated that particles with a mass median equivalent diameter (MMED) of less than 5 μm account for 80% of the manganese in suspended particulate matter, while particles with an MMED of less than 2 μm are responsible for 50% of this manganese. It is unclear if these results apply to particles in urban or rural locations. It is known, however, that the size of manganese particles in the air tends to differ depending on the source; big particles tend to predominate around mining operations, whereas small particles tend to predominate around ferromanganese and dry-cell battery industries (WHO, 1999). A mean manganese content of 1338 mg/kg was found in airborne particles (>2 μm) collected over the seas (Lee & Duffield, 1979).
Widespread airborne dispersal would be anticipated based on these results (IPCS, 1981). While manganese deposition showed little spatial variation between urban and rural areas, Fergusson & Stewart (1992) reported that manganese concentrations in New Orleans, Louisiana, USA, increased four times between rural and urban areas. This is in contrast to other metals that are deposited in different ways, such as copper, lead, cadmium, and zinc. On manganese’s atmospheric reactions, very little information is known (USEPA, 1984). Manganese can react with nitrogen dioxide and sulphur dioxide; however, it has not been shown that these reactions happen in the atmosphere. Manganese’s arithmetically averaged annual wet flow was 1900 μg/m2 for a sea loch in Scotland and 1190 μg/m2 for the USA’s Chesapeake Bay (Scudlark et al., 1994; Hall et al., 1996). Manganese deposition rates in the western Mediterranean Sea (northwest Corsica) during 1985-1987 varied from 0.0023 to 0.0072μg/cm2 daily. The greatest manganese atmospheric deposition was caused by sporadic but powerful Saharan sandstorms (Remoudaki et al., 1991). The manganese deposition rate for Burnaby Lake, British Columbia, Canada, was determined to be 350 μg/m2 per day. For the whole watershed, the anticipated yearly position rate was 7.7 tons (Brewer & Belzer, 2001). There are two primary types of manganese found in aquatic environments: Mn(II) and Mn(IV). Oxidation and reduction processes, which can be abiotic or microbially driven, mediate the change between these two forms (Nealson, 1983; Thamdrup et al., 2000; Heal, 2001). pH and redox circumstances have a major role in the environmental chemistry of manganese; Mn(II) predominates at lower pH and redox potential, and in non-dystrophic waters, the fraction of colloidal manganese oxy-hydroxides increases above pH 5.5 (LaZerte & Burling, 1990). Dissolved manganese concentrations in waterways receiving acid mine drainage varied from 250 to 4400μg/litre below pH 3, but were less than 40 μg/litre above pH 5.5 (Filipek et al., 1987). Cherry et al. (2001) found that manganese concentrations in sediment dropped from 400 mg/kg at pH 5.6-5.9 to 8 mg/kg below pH 3. This decline was attributed to manganese dissolution affected by acid mine drainage. When Mn(II) is present in anaerobic conditions, a complicated series of oxidation/ precipitation and adsorption events take place, ultimately rendering the manganese as insoluble manganese dioxide physiologically inaccessible. However, in waters with a pH lower than 8.5, the kinetics of Mn(II) oxidation are sluggish (Zaw & Chiswell, 1999). Days can pass in natural water before manganese oxidizes and precipitates, but years can pass in artificial water (Stokes et al., 1988). However, as pH rises or when catalytic surfaces like manganese dioxide are present, manganese oxidation rates rise as well (Huntsman & Sunda, 1980). Rapid manganese oxide oxidation and precipitation occurred in a stream receiving manganese-rich inflows due to accident drainage (Scott et al., 2002). The series of events that precipitate as manganese dioxide after Mn(II) is oxidized involves the simultaneous occurrence of multiple manganese forms (e.g., dissolved Mn(II), hydrous oxides of Mn(III), Mn(II) adsorbed to particulates, and Mn (II) ligand complexes), each of whose concentration is influenced by a variety of variables such as pH, organic and inorganic carbon, sulphate, chloride, temperature, and time (Stokes et al., 1988). Both chemically and bacterially reducing Mn(IV) to the Mn(II) oxidation state is possible in low oxygen groundwater (Jaudon et al., 1989). Manganese is relatively weakly linked to dissolve organic carbon, and there is minimal evidence of organic connections between manganese and natural fluids (L’Her Roux et al., 1998). Therefore, manganese speciation in natural waters is not significantly influenced by organic complexation. Even with significant amounts of naturally occurring dissolved organic carbon, field research has shown that organically bound manganese is negligible (Laxen et al., 1984). Manganese will often become more accessible when pH and redox potential decrease because the Mn(II) ion is more soluble than the Mn(IV) ion (Heal, 2001). Manganese solubility can be increased by the presence of chlorides, nitrates, and sulphates, which will boost aqueous mobility and plant absorption (Reimer, 1999). Manganese speciation in tropical north Australia’s Magela Creek was investigated by Hart et al. (1992). They postulated that equilibrium between Mn(II) and oxidized species may be achieved during the typical residence period of water in the stream at higher temperatures (30 °C) and greater rates of bacterially induced oxidation. Colloidal manganese has the potential to dominate speciation through this method. There is proof that surface waters now contain higher quantities of manganese due to upland regions being reforested. Mean manganese concentrations and the amount of conifer cover in the watershed are significantly positively correlated, according to an analysis of sites in the United Kingdom conducted between 1988 and 1996 (Heal, 2001).
Increased manganese concentrations result from the minerals being taken up by the trees from the environment through foliar leaching and wash-off of manganese in fine mist and dry particles (Shanley, 1986; Heal, 2001). Conifer plantation litter may potentially accelerate the leaching of manganese from the soil into runoff. There is ample evidence linking increased manganese concentrations in surface waters to soil and water acidification in catchments covered with conifers (Heal, 2001). Soil type and catchment hydrology affect how much land use affects manganese concentrations in upland catchments (Heal, 2001; Heal et al., 2002). According to Heal et al. (2002), the summer-autumn hydrological transition and summer base flow are crucial times for higher manganese concentrations in run-off. Manganese will not become dominant in chemical processes and the system will reach equilibrium speciation until it enters lakes, estuaries, and the ocean, where residence durations are significantly longer (Laxen et al., 1984). In rivers, manganese is frequently adsorbed to suspended particles.
The majority of the manganese in the Paraiba do Sul-Guandu River, Rio de Janeiro, Brazil, that came from industrial sources (metallurgical and chemical industries), was attached to suspended particles Malm et al., 1988. For a wide range of UK rivers, a positive association has been documented between manganese concentrations and suspended sediment levels Laxen et al., 1984; Neal et al., 1998, 2000. Soluble manganese compounds’ propensity to adsorb to soils and sediments can vary greatly, primarily based on the soil’s organic content and cat ion exchange capacity Hemstock & Low, 1953; Schnitzer, 1969; McBride, 1979; Curtin et al., 1980; Baes & Sharp, 1983; Kabata Pendias & Pendias, 1984. The “particulate” and “dissolved” phases of rivers and streams may become dissociated from weathering processes, according to Laxen et al. (1984). This might result in suspended sediment and influxes of Mn(II) species leaching from anoxic soil and ground waters.
Study Area
Abeokuta: Abeokuta, the capital city of Ogun state is the largest city in the southwestern region of Nigeria. It is located on the eastern bank of Ogun River, with its centre located at 7oN & 3oE, 48 mols (71 km) North of Lagos by railway, 81 miles (130km) by water. Olumo Rock, a granite outcropping is located at the city centre and is of course the site of traditional celebrations with a population of over 500, 000 people.
Ibadan: Ibadan is the capital city of Oyo state. It is also the third largest metropolitan area; Ibadan is the largest and most populous city with a population of 1,338,659. It is also located in the southwestern part of Nigeria, 128km inland northeast of Lagos and 530km southwest of Abuja.
Sampling Sites: The soil samples were collected from eight different land uses in different areas in the two locations, Ibadan and Abeokuta respectively. The eight land uses include crop farms, animal farms, educational areas, residential areas, roadside, industrial areas, markets, and forests in Abeokuta and Ibadan. The selected areas of both locations are described in the tables below.
Sample collection: A total number of 96 soil samples were collected from the selected areas. The soil samples were collected based on two depths (0-20cm and 20-40cm deep, respectively), three replicates, from different spots in each area of the two locations, Abeokuta and Ibadan respectively. The samples were collected using soil auger. The coordinates of each position of areas in which the samples were taken with the Global Positioning System (GPS) tool (Table 1).
Note: FUNAAB- Federal University of Agriculture Abeokuta
MAPOLY-Mashood Abiola Polytechnic
Sample Preparation: The soil samples were air-dried; the impurities such as stones and tree leaves were removed from them and then passed through a 2mm sieve.
Sampling Analysis: The digestions of the samples were done, and the concentration of the toxic element (Nickel) was measured using Atomic Absorption Spectrophotometry, AAS (Burt, 2004). Soil analysis of other soil parameters including soil pH, phosphorus content, electrical conductivity, nitrogen content and particle size was done (Table 2).
Procedure for Determination of Electrical Conductivity
1) Gather a fresh soil sample in a plastic zip-loc bag. Try to get a
profile from the top 6” of soil that the plants will grow in and take
care not to contaminate the sample by touching anything.
2) Open the bag and let it air-dry for a few hours until it is
mostly dried.
3) Mix the soil in the bag to ensure a homogenous sample and
then use a sieve with approximate 2mm spacing to remove any
large soil clumps.
4) Measure out ½ of a cup of the dried soil and put it into a
glass beaker.
5) Measure out ½ of a cup of distilled water and put this into
the glass beaker with the soil.
6) Stir the mixture gently for 30 seconds. Do not mix too harshly
as you may destroy the humus structure and the soil may give
up elements that it otherwise would not do in nature. Let the
soil-water suspension stand for 30 minutes.
7) Stir water gently again before taking the EC measurement.
Insert the EC meter into the beaker and swirl it gently around in
the soil-water extract. After approximately 30-60 seconds or after
the EC reading has stabilized, read the digital display on your meter.
Nitrogen Determination Procedure
Digestion:
1) Fill a digestion flask with about 1 g of the ground material, and note the weight (W) to the closest 0.1 mg. As a way to verify that the digestion settings are accurate, add reagent blank and high-purity lysine HCl. For the purpose of determining dry matter in a lab, weigh a second subsample. 2) Include 15 grams of potassium sulphate, 0.04 grams of anhydrous copper sulphate, 0.5 to 1.0 grams of alundum granules, or 16.7 grams of K2SO4, 0.01 grams of anhydrous copper sulphate, 0.6 grams of TiO2, and 0.3 grams of pumice. Add 20 mL of sulfuric acid after that. In case the sample weight exceeds 1 g, add 1.0 mL sulfuric acid for every 0.1 g of fat or 0.2 g of other organic stuff. 3) Set the flask on the burner that has been prepared; adjust so that it will boil 250 mL of water at 25 °C for five minutes. 4) Swirl slowly and heat for a further 90 minutes for copper catalyst or 40 minutes for CuSO4/ TiO2 mixed catalyst, or until white fumes clear the flask’s bulb. 5) Allow it cool, then slowly stir in 250 mL of distilled water to bring it down to room temperature (less than 25 ℃). Note: You can add more water to the distillation, up to around 275 mL, if bumping happens.
Distillation:
1) To prepare the titration flask, fill it with water to the right
level so that the condenser tip is submerged. If you are not sure
how much acid to use, try 15 mL of it and 70 mL of water. Pour
around 85 mL of water into a pipette containing 1 mL of acid to
create the reagent blank. Add three to four drops of the indicator
solution methyl red.
2) To lessen foaming, add two to three drops of tributyl citrate
or another antifoam agent to the digesting flask.
3) Include an additional 0.5–1.0 g of alundum granules.
4) Add enough 45% sodium hydroxide solution (about 80 mL)
slowly down the side of the flask to make the mixture highly alkali.
(To avoid losing ammonia, do not combine until the flask is
attached to the distillation device.)
5) Attach the flask to the distillation apparatus right away, and
distil until at least 150mL of distillate is collected in a titrating
flask at a rate of roughly 7.5 boil rate (temperature adjusted to
bring 250mL of water at 25oC to boil in 7.5 min).
6) Take out the titrating and digestion flasks from the unit. As
you remove the flasks, rinse the condenser tube with distilled
water.
Titration: Record the volume to the closest 0.01mL (VNaOH) and titrate surplus acid with standard sodium hydroxide solution to the orange endpoint (colour shift from red to orange to yellow). Analogously, titrate the reagent blank (B).
Determination of Organic Matter
The following methods are used to identify organic matter:
1) Transfer 1 gram of dirt into a 500 millilitre Erlenmeyer flask.
2) Add a 1N potassium dichromate solution (10 millilitres).
3) Add 20 ml of sulfuric acid, and stir slowly for a minute, being
careful not to propel soil up onto the flask’s edges. Give it a halfhour
to stand.
4) Use deionized water to dilute to 200 millilitres.
5) Include 10 drops of diphenylamine indicator, 0.2 grams of
ammonium fluoride, and 10 millilitres of phosphoric acid.
6) Titrate the solution with 0.5N ferrous ammonium sulphate
until the colour turns turbid blue from dull green. Drop by drop,
add the titrating solution until the colour changes to a vivid green,
which indicates the endpoint.
7) Use the same procedures to prepare and titrate a blank.
8) After each set of samples is examined, prepare one duplicate
sample and one quality control sample.
The Recommended Procedure for the Determination of Soil pH
Wash the electrodes and the cup well after calibrating the equipment. To get a rough estimate of the pH, fill the cup partially with the solution to be tested. This value is generally considered to be an estimate and will wander. The pH values obtained from taking numerous readings on the same solution will become increasingly stable with time. For well-buffered solutions, three parts could be enough to provide pH readings that exhibit drifts of less than ± 0.04 unit in one or two minutes and are repeatable to ±0.04 unit. Up to six parts of the test solution could be needed for extremely diluted or unbuffered solutions, and the pH results might drift and only be repeatable to ± 0.05 unit. To achieve a precision of more than 0.1 pH unit, the temperature of the test solutions, glass and calomel electrodes, standard solutions, and wash water must all be within 2 °C of one another. Additionally, the electrodes, standard solutions, test solutions, and wash water must all be maintained at the measurement temperature for a minimum of two hours prior to the measurement in order to minimize the effects of thermal or electrical hysteresis on the electrodes (Table 3).
Note: FUNAAB- Federal University of Agriculture Abeokuta
MAPOLY- Mashood Abiola Polytechnic
5 g of air-dried soil should be weighed, and in an extraction cup, 25 ml of 2NH4F + 25 ml of 2N HCl reagent should be measured. You may either shake for five minutes on a mechanical shaker or stir for one minute. Pipette the sample (8 ml) into a pair of cups. Mix well after adding 5 drops of phosphorus ammonium molybdate solution. Next, thoroughly mix with 5 drops of the phosphorus C reagent. After letting the solution remain for half an hour, measure the wavelength at 660 millimetres on the colorimeter. After taking measurements, use the standard curve to calculate P values (Table 4).
Note: FRIN- Forestry Research Institute of Nigeria UI- University of Ibadan NIHORT- National Horticultural Research Institute
Data Analysis
Manganese and other soil parameters were correlated after statistical analysis using Analysis of Variance (ANOVA) and Pearson’s rank correlation (data with normal distribution) was completed.
In the table below, it was observed that there was no significant difference (P ˃ 0.05) between soil depths (0- 20 and 20-40 cm) in electrical conductivity in Abeokuta and manganese in both Abeokuta and Ibadan soils. However, soil depth of 20- 40cm had significantly higher manganese content than 0- 20cm in Ibadan soil. This suggests that depth did not affect the distribution of manganese in Abeokuta and Ibadan soils (Table 5) . The values of manganese in Ibadan are relatively greater than that of Abeokuta. There is also evidence of manganese movement down the profile in Ibadan. In Table 6, it was observed that there was no significant difference (P ˃ 0.05) between land uses (crop farm, animal farm, education, forest, industrial, residential, market, roadside) in electrical conductivity in Ibadan except for Abeokuta where there was significantly different between land uses in electrical conductivity. There was no significant difference (P ˃ 0.05) between land uses in manganese content in both Abeokuta and Ibadan soils. However, the values of manganese concentration in Ibadan are relatively higher than that of Abeokuta with industrial, forest and residential areas with the highest values.
Table 5: Effect of soil depth on manganese concentration and the electrical conductivity of Abeokuta and Ibadan soils.
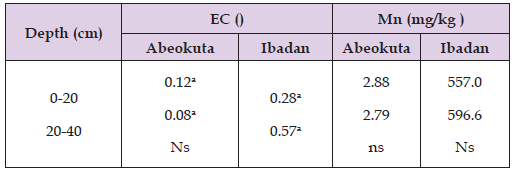
Table 6: Effect of land use on manganese concentration and the electrical conductivity of Abeokuta and Ibadan soils.
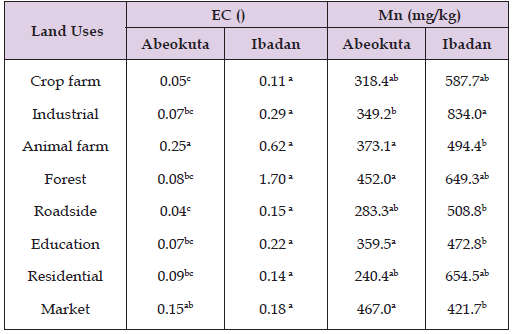
These denote that land use affected the distribution of manganese content in some soils of both Abeokuta and Ibadan. The correlation coefficients shown in Table 7 indicated that soil manganese is positively correlated with most of the other heavy metals at both locations except pH, silt and clay in Abeokuta (-0.15, -0.31, -0.17, P ≤ 0.01). In Table 8, the table shows how the value content of electrical conductivity and manganese concentration were affected by the different factors including the soil depths, the locations (Abeokuta and Ibadan), and the land use. The soil depths did not affect both the electrical conductivity and manganese content as there are no significant differences between the values of electrical conductivity and manganese content respectively. There are significant differences between the values of manganese content under the factor of locations in some soils of both Abeokuta and Ibadan but there was no significant difference between the values of electrical conductivity in some soils of both Abeokuta and Ibadan soils.
Note: *, ** significant at P<0.05 and <0.01 respectively
Table 8: The effect of soil depth, location and land use on the distribution of electrical conductivity and manganese in both of the locations (Abeokuta and Ibadan).
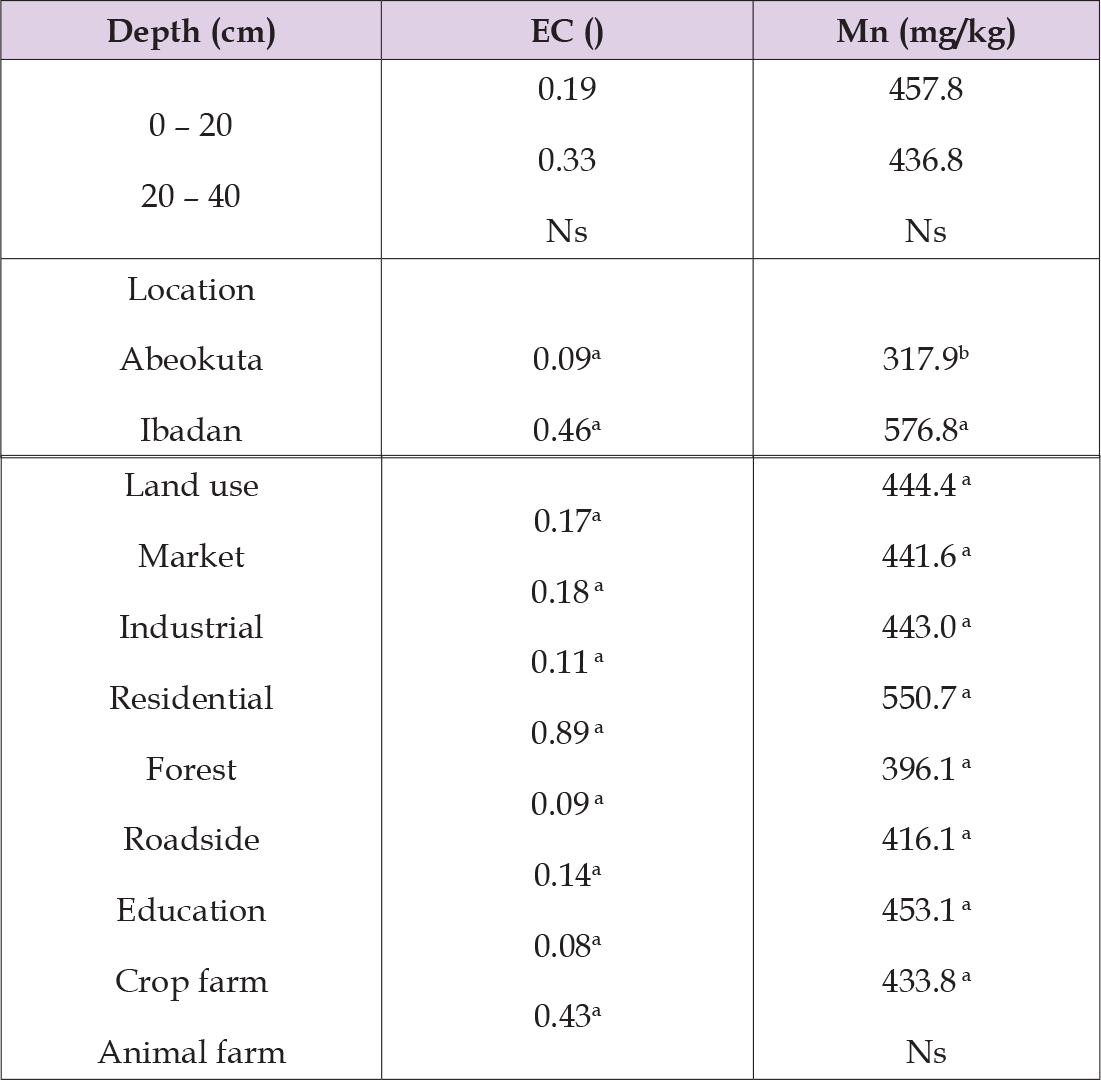
This denotes that the locations have an effect on the distribution of manganese concentration but do not have an effect on its electrical conductivity. In comparing both locations, that is Ibadan and Abeokuta respectively, Ibadan has the highest values of manganese. The correlation coefficients shown in the table above indicated that the soil manganese is positively correlated with other heavy metals at both locations; and soil manganese positively correlated with most of the other soil parameters (Sand, N, C, P at P < 0.05 and EC at P < 0.01). There are also significant negative correlations between soil nickel and pH, silt and clay (P < 0.01) in both locations (Table 9).
Table 9: Correlation between nickel and other soil parameters in both the Abeokuta and Ibadan soils.
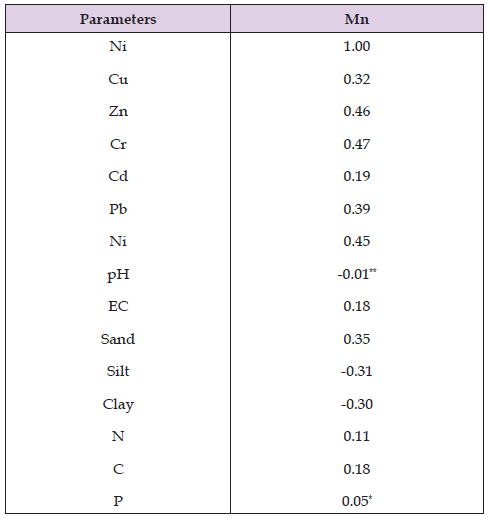
Note: *, ** significant at P<0.05 and <0.01 respectively
After the analysis, and from the result land use effect on Manganese concentration should that there is a significant difference in the values of land use although crop farm, forest, and residential land use are not significantly different from each other so also, animal farm, roadside, education and market area are also not significantly different from each other, however, the values of manganese concentration in Ibadan is significantly different from each other, however, the values of manganese concentration in Ibadan is higher than that of Abeokuta most especially in industrial areas. Forest and residential areas also have high values of manganese concentration in Ibadan. This must be because Ibadan has more industries than Abeokuta most especially production industries e.g. 7 up companies. The electrical conductivity of soils in Ibadan are not significantly different from each other while it differs in Abeokuta as industrial, forest, education and residential areas are not significantly different from each other while crop farm and roadside are also not significantly different from each other, however, animal farms have the highest values of electrical conductivity.
The downward mobility of manganese in Ibadan is high however in some soils of Abeokuta; it appears that there is no downward mobility of electrical conductivity. The electrical conductivity of some soils in Abeokuta and Ibadan is not significantly different from each other. The average manganese soil concentration is 40-900 mg/kg. The soils analysed fell in the range of average manganese soil concentration in natural soil and this shows that the soils are not contaminated although this is no proof that other heavy metals are not contaminating the soils. Correlation with other soil parameters most especially soil pH, soil organic matter, particle size and phosphorus of the analysed soils shows the relationship with each other.
The analysis revealed that land use affected the distribution of the manganese in some soils of both Abeokuta and Ibadan. Manganese concentration in Ibadan is very high in the industrial areas almost reaching the maximum range of tolerable levels in natural soils. Agriculture might be difficult to practice in these areas as it can be toxic since manganese is needed in trace quantity for plant growth. Its toxicity alters physiological, biochemical and molecular processes at the cell level. The downward mobility of electrical conductivity in both soils is high and it is an indication of the amount of nutrients available for crop absorption. It aids less use of fertilizer though weed control will be more. Electrical conductivity measurements are consistently correlated to soil properties that affect soil productivity including soil texture, cat ion exchange capacity, and organic matter. It is a great prediction of soil health.


