Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Karolina Juszczak and Krzysztof Walczak*
Received: April 18, 2024; Published: April 30, 2024
*Corresponding author: Krzysztof Walczak, Department of Organic Chemistry, Bioorganic Chemistry and Biotechnology, Faculty of Chemistry, Silesian University of Technology, 44-100 Gliwice, Poland
DOI: 10.26717/BJSTR.2024.56.008847
Altered glucose metabolism is characteristic of many cancer cells, especially in the hypoxic zone of solid tumours, which is associated with a poor prognosis. Hexokinase (HK) is an enzyme involved in the first step of glycolysis, the phosphorylation of 6-OH in glucose molecules. Hexokinase 2 (HK2) is one of the isoforms overexpressed in cancer cells. In addition, HK2 binds to the voltage-dependent anion channel 1 (VDAC1) located on the outer mitochondrial membrane, protecting the cancer cell from apoptosis. Targeting HK2 shows promise for a novel therapy in cancer treatment. HK2 inhibitors inhibit glycolysis, reduce the energy supply to cancer cells, and disrupt the function of the HK2-VDAC1 complex, thereby inhibiting cancer growth and development. According to recent studies, the use of HK2 inhibitors together with known antitumour drugs in combined therapy improves efficacy in solid tumour treatment. This may be colligated to the susceptibility of the hypoxic cells to anaerobic glycolysis and their resistance to anticancer therapies such as chemotherapy and radiotherapy. This review describes the function and expression of HK2 in cancer cells and the differences between HK2 and other isoforms. We present systematized reported HK2 inhibitors with clinical potential in the treatment of cancer and indicate that the main limitation of HK2 inhibitors usage is low selectivity HK2 inhibitors and consequently their cytotoxicity. The present study reconfirms a strong necessity to develop new effective and selective HK2 inhibitors.
Abbreviations: HK : Hexokinase; ATP: Adenosine-5′-Triphosphate; PTP: Permeability Transition Pore; VDAC: Voltage-Dependent Anion; PT: Permeability Transition; MCT: Monocarboxylate Transporter; MPC: Mitochondrial Pyruvate Carrier; EE: Energy Expenditure; MH: Mannoheptulose; REF: Embryonic Fibroblasts; HTS: High-Throughput Study; ROS: Reactive Oxygen Species; NSCLC: Non-Small Cell Lung Carcinoma; MJ: Methyl Jasmonate; LOX: Lipoxygenase; FAO: Food and Agriculture Organisation; WHO: World Health Organisation; SIRNA: Small Interfering RNA; SHRNA: Small Hairpin RNA; HNIS: Human Sodium Iodide Symporter; PLD: Potentially Lethal Damage; LD: Lonidamine
Cancer cells are characterized by changing in cellular metabolism as compared to normal cells. Reprogramming of glucose metabolism is one of the characteristic features of the metabolic disorder in cancer cells. Accelerated glucose metabolism is required to supply enough high-energy intermediates necessary to support biosynthetic processes in rapidly proliferating cancer cells [1,2]. High glucose consumption causes an increasing level of lactate excretion in cancer cells even in the presence of oxygen (Figure 1). This phenomenon is known as the “Warburg effect” or “aerobic glycolysis” [3]. Aerobic glycolysis is a less energy-efficient metabolism than oxidative phosphorylation. A higher rate of glycolysis in cancer cells in comparison to their healthy cells has been observed in many types of cancer, including colon, breast, lung, pancreatic, head and neck, prostate cancer, melanoma, and glioblastoma [1-4]. This abnormal glucose metabolism may be explained by the increased demand for precursors for the synthesis of cell components like fatty acids, amino acids, and nucleic acids in rapidly growing cell tumours [5]. Glycolysis of glucose to pyruvate involves several enzymes. Inhibition of these enzymes can retain the generation of energy in cancer cells. A key role plays hexokinase (HK), an enzyme participating in the first limiting stage of glycolysis, the phosphorylation of glucose to glucose-6-phosphate (G6P) (Scheme 1). Glucose-6-phosphate opens the anabolic activity in cancer cells [6]. Hexokinase, due to its key role in glycolysis is considered, among others, as an important target for anticancer therapy.
There are four mammalian hexokinase isozymes with different subcellular locations, physiological functions, and glucose affinity [7- 9]. These are hexokinase 1 (HK1), hexokinase 2 (HK2), hexokinase 3 (HK3), and hexokinase 4 (glucokinase, HK4). HK1 interacts with the outer membrane of mitochondria [10,11]. HK2 is usually placed on the outer membrane of mitochondria but is also located in the cytosol [10,12]. Both HK1 and HK2 bind to the outer mitochondrial membrane through a N-terminal hydrophobic region [11-13]. These two isoforms are structurally like each other. HK1 and HK2 are in 73% identical and 87% similar [9]. HK3 is present in the perinuclear compartment [8,14], however, HK4 operates in the cytosol [13,14]. HK1, HK2, and HK3 have two similar homologous with molecular masses of 50 kDa with high affinity for glucose (Table 1) [10,15]. The reaction product-G6P inhibits by negative feedback three isoforms namely HK1, HK2, and HK3 [10,15]. An elevated level of inorganic phosphate (PO43-) also inhibits HK1, HK2 and HK3. In the case of HK1 was observed that a low level of PO43- stimulating this isoform [16]. HK4, called glucokinase, has 50 kDa domains and has a lower affinity for glucose (Table 1) [10]. Isoforms are different by the number of catalytic domains, HK1 and HK3 have a single active catalytic domain, while HK2 and HK4 have two active sites [15,17]. All hexokinase isoenzymes have other mechanisms of regulation that lead to different distribution in tissues and cell types [8-18] (Table 1).
Among the four isoforms of hexokinase, HK2 is rarely expressed in normal tissues, except for some insulin-sensitive tissues. In contrast, this hexokinase isoform is overexpressed in tumour cells [12,14,19]. HK2 is required for the initiation and maintenance glycolysis process (Patra, et al. [20]) showed that HK2 deletion in the mouse models of KRas-driven lung cancer and ErbB2-driven breast cancer is therapeutic for cancer. Furthermore, elevated levels of HK2 are significantly associated with some tumour aggressiveness, promoting the development of metastasis in some rapidly growing tumours or resistance in therapy [4,12,14,15,19,21,22]. HK2 is a marker for poor prognosis tumours such as breast, lung, prostate, glioblastoma, hepatocellular, gastric, and liver cancer [4,15,19,21,22]. In solid tumours, there are hypoxia zones where oxygen levels are much lower than in normal tissues. Reprogramming the cancer metabolism allows maintenance of the redox homeostasis associated with hypoxia-induced factors such as HIF-1α. HIF-1α promotes the transcription of several genes, including the HK2 gene encoding. HIF-1α and HK2 overexpression are associated with a lethal cancer phenotype characterized by invasion and metastasis, and resistance to chemotherapy and radiation therapy [23,24]. Sparse reports suggest a connection of HK1 with cancer cell growth [25,26].
Mitochondria are intracellular multifunctional organelles present in eukaryotic cells. They engage in energy production, cell metabolism, and programmed cell death. The key role of mitochondria is the production of adenosine-5′-triphosphate (ATP), a key source of energy and phosphate groups in a cell. The mitochondria in cancer cells differ from normal mitochondria; they prefer changes in bioenergetics and biosynthesis shifting metabolic processes from oxidative phosphorylation to anaerobic glycolysis. It is assumed that the Permeability Transition Pore (PTP) complex in mitochondria regulates the release of pro-apoptotic factors. Furthermore, regulation of the PTP opening protects against the loss of membrane potential, leading to the disintegration of the outer mitochondrial membrane. The regulation of PTP components is different in cancer cells. Studies have shown that the PTP complex is associated with components such as HK2 and Voltage-Dependent Anion (VDAC), a transporter of mitochondrial metabolites, which are specific for cancer (Figure 2) [27].
Mitochondria-associated HK2 plays a crucial role in the aerobic glycolysis of tumour cells [15]. HK2 binds to the voltage-dependent anion channel 1 (VDAC1) located on the outer mitochondrial membrane [15,25,28,29]. VDAC1 allows the diffusion of ATP between mitochondria and cytosol; in this way, cell HK2 has direct access to intramitochondrial ATP used for glucose phosphorylation. The binding of HK2 to VDAC1 allows the defection of G6P, the phosphorylation product, resulting in protection from inhibition by negative feedback [28]. The interaction of HK2 with the outer membrane of the mitochondria forces cancer cells to have a high rate of glycolysis [14,28]. Mitochondria- associated HK2 cooperation with VDAC1 also interferes with the cell’s apoptotic pathways by preventing the delivery of apoptotic factors to the mitochondria and the release of apoptotic regulators by mitochondria to the cytoplasm (such as cytochrome c), protecting the tumour cell from apoptosis [15,25]. VDAC1 is often overexpressed in many types of cancer [9,25].
The intracellular environment has a strong influence on HK2 activity and its association with the outer mitochondrial membrane. The increase a glucose uptake interferes with the induction of glucose transporter (GLUT) expression hence increasing hexokinase activity. Another factor, such as protein kinase B (Akt), plays a key role in the localization and regulation of HK2 activity. Activation of this factor induces the activity of the hypoxia-inducible transcription factor HIF-1α that promotes the expression of GLUT and HK2 [30]. Other factors regulating HK2 expression are c-MYC, protein p53 mutations, and insulin [15]. Protein kinase B, known as Akt, affects different signaling pathways and engages in glucose metabolism in cancer cells. The Akt protects cells from apoptosis by maintaining normal mitochondrial function and the correct state of PTP opening, which maintains hexokinase association with the outer mitochondrial membrane (Gottlob, et al. [31,32]). demonstrated that activated Akt increases mitochondria-associated hexokinase activity by approximately 50%. It has been confirmed that Akt causes HK2 disconnection from mitochondria and sometimes translocation of HK2 from the mitochondrion to the nucleus [30]. Magalhães, et al. [10] investigated changes in HK2 intracellular localization from mitochondria to cytosol under different concentrations of G6P, glucose, and Akt. Another study confirmed that the translocation of HK2 is dependent on its enzymatic activity [33].
Competitive and/or Non-Competitive HK2 Inhibitors
Several potential HK2 inhibitors that directly affect HK2 activity (Figure 3) have been described in the literature [9,29]. They have shown insufficient effectiveness and limited selectivity in their practical application in cancer treatment. The search for new potential anticancer drugs requires further and more thorough and further research in the HK2 inhibitors field. Herein, the most promising HK2 inhibitors that mimic G6P binding or bind to the glucose binding site are described (Figure 3).
Metformin (Met): Metformin (Met) is a biguanide derivative used in therapy for type 2 diabetes. Met, like a drug approved by the FDA, is characterized by its safety profile. Common side effects of Met include gastrointestinal complaints and, very rarely, lactic acidosis. Moreover, long-term metformin treatment leads to vitamin B12 deficiency [34]. According to the meta-analysis, patients treated with Met showed a reduced risk of cancer incidence and mortality [35]. To date, several mechanisms of Met action have been demonstrated. One is that Met occupies the binding site of the phosphorylation product, G6P, changing the conformation of HK2 and directly reducing HK2 activity. Additionally, it also indirectly dissociates the enzyme from the mitochondria, leading to the activation of apoptotic cell death [36]. The study of (Salani, et al. [37]) demonstrated that Met inhibits (at a mM concentration of 0.0375 mM to 10 mM) both purified HK1 and HK2 as well as cell lysates in a dose-dependent fashion but does not have any effect on HK4. Preclinical studies have revealed that Met has the efficacy of standard chemotherapy in a variety of cancer cells and accelerates tumour regression and prolonged remission as an effect of combined treatment with Met and individual chemotherapeutic agents [38].
Lonidamine (LD): Lonidamine (LD) 1-(2,4-dichlorobenzyl)- 1-H-indazol-3-carboxylic acid is a known antispermatogenic agent with anticancer properties. (Florldl, et al. [39,40]) reported LD as an HK2 inhibitor. LD directly inhibits HK2 activity and interferes with HK2 binding of HK2 to VDAC. Tests performed on Ehrlich ascites tumour cells indicated that 20 μM LD reduced more than 50% amount of G6P formed by mitochondria, which is related to the effect on mitochondrial HK2. The antitumour activity of LD was assessed in Cholangiocarcinoma cell lines with characteristic overexpression of HK2. LD significantly suppressed the growth of Cholangiocarcinoma cell lines with an average IC50 value of 137 μM [41]. LD exhibits selective intracellular activity against tumour cells, resulting in low toxicity to normal cells, provided the doses do not exceed 400 mg/m2 (intravenous and oral) [42]. Due to its promising anticancer effects, clinical studies were performed using LD. A phase II clinical trial with LD was conducted in the treatment of malignant glioma that overexpresses HK2.
The data obtained confirmed limited therapeutic activity, 2 responses, and 3 stable diseases obtained in the 10 evaluable patients [43]. Clinical research on LD has demonstrated that combination of LD with chemotherapy and radiation is safe and potentially effective [44]. LD treatment does not cause the typical side effects of traditional anticancer drugs such as germ cell mutation, alopecia, and gastrointestinal mucosal necrosis. Phase II clinical trials demonstrated that the most common side effects of oral LD were myalgia, testicular pain, and lethargy [45]. The LD is used in few European countries for the treatment of cancer as a glycolysis inhibitor but has not yet been approved by the FDA [46]. Although LD tends to extend median survival in cancer patients with low toxicity, more clinical trials are necessary to determine the toxicity and benefits of this drug.
Recent research has shown that the mechanism of LD’s anticancer action is not just through HK2 inhibition. LD has multiple targets activity, such as Permeability Transition (PT) pore complex, Monocarboxylate Transporter (MCT), Mitochondrial Pyruvate Carrier (MPC), and anion channel VDAC [47]. The modification of lonidamine obtained by combining lonidamine with 7-hydroxy-4-methylcoumarin led to the discovery of a new promising anticancer agent (Figure 4). Measurements of the cytotoxic effect of LD and hybrid lonidamine- coumarin in the MCF-7 and A549 cell lines gave IC50 values for LD and hybrid lonidamine-coumarin as 239.4 μM, 33.12 μM for MCF-7 and 444 μM and 222.8 μM for A549, respectively. Based on molecular docking studies, a good binding affinity to HK2 was identified for hybrid lonidamine-coumarin, so this may confirm HK2 as a molecular target for this agent [48].
2-Deoxy-D-Glucose (2-DG): As early as the 1950s, 2-DG was proven to inhibit anaerobic glycolysis [49]. 2-DG is a glucose analog in which the hydroxyl group at position 2 is replaced by hydrogen. 2 DG is a glucose mimetic, so it has a high affinity for hexokinase (Km=27 μM) and competes with glucose for the active site of HK [49]. The mechanism of the 2-DG antitumour action involves inhibition of anaerobic glycolysis (Figure 5). It is known that cells relying on glycolysis are more susceptible to 2-DG. Overexpression of glucose transporters and glycolytic enzymes in cancer cells increases 2-DG uptake in cancer cells compared to normal cells. 2-DG is non-toxic to humans and animals. The safety of 2-DG as a single agent for a dose of 63 mg/kg/day has been confirmed in clinical studies [50]. However, the administration of 2-DG for a long time in high doses could be toxic, as it reduces glucose consumption in normal tissues, especially the brain. The most common reported side effect is reversible hyperglycemia [50]. Due to its efficient cytotoxicity in a wide variety of tumour cells, 2-DG has the potential for use in anticancer therapy and combination therapy with standard chemotherapeutics as confirmed by clinical studies [5]. However, the poor pharmacokinetic and pharmacodynamic profile of 2-DG is a major reason for the lack of 2-DG therapeutic activity. To improve 2-DG activity, its derivatives were synthesized. 3,6-Di-O-acetyl-2-deoxy-D-glucose possesses improved pharmacokinetic and pharmacodynamic properties, allowing two orders of higher maximum concentration of 2-DG in plasma cells compared to an equal molar dose of pure 2 DG [51]. The 2-DG derivative, namely, 2-[18F]-fluoro-2-deoxy-D-glucose has been used in positron emission tomography (PET) as a diagnostic agent in cancer. Interestingly, during the pandemic in recent years, 2-DG was approved in India for the treatment of patients with COVID-19 in severe cases [52].
Other Glucose Analogs: (Machado de Domenech, et al. [53]) conducted studies on the effect of glucose analogs on hexokinase. In this research, they used isozymes 1 and 2 of yeast hexokinase and brain hexokinase and studied the affinity for the enzyme and the rate of the phosphorylation reaction. Mannosamine and 5-thioglucose (5-TG) were shown to be the most promising competitive inhibitors [53]. 5-TG is a D-glucose derivative in which the sulfur atom replaced the oxygen in the ring and was first synthesized in 1962 [54]. 5-TG was found to inhibit glucose phosphorylation and is slowly phosphorylated. The reaction of 5 TG phosphorylation by hexokinase is not as effective with Vmax 0.3% in comparison to glucose [53]. Another research group confirmed that 5-TG as a competitive inhibitor has a moderate affinity for brain hexokinase with a similar Ki of 0.17 mM [55]. They also showed that this compound is an ATP inhibitor at the active site of the HK with estimated Ki values of 0.31 and 0.48 mM. In addition, 5-thioglucose phosphorylation gives 5-thioglucose 6 phosphate.
It was evaluated as glucose-6-phosphate analogs to inhibit soluble (HK1) and mitochondria-bound hexokinase (HK2). 5-Thioglucose- 6-phosphate at a concentration of 2.5 μM inhibited mitochondria- bound soluble hexokinase HK1 and HK2 mitochondria-bound hexokinase by 60% and 20%, respectively [55]. Mannoheptulose (MH), a 7-carbon monosaccharide, is a nonselective, competitive, and non-competitive inhibitor of various isoforms of hexokinase such as HK1, HK2, and HK4 [56,57]. However, on the other hand, the safety profile for animal treatment with MH is satisfactory. In studies of Energy Expenditure (EE) in healthy adult dogs, MH was used as an avocado extract. All dogs showed good general health without side effects [58].
(Al-Ziaydi, et al. [59]) conducted an in vitro study on the proliferation of the effect of MH on breast cancer cells (AMJ13 and MCF7) due to inhibition of the biochemical pathway. They determined IC50 for AMJ13 and MCF7 using the MTT cytotoxicity assay with values of 124,7 μg/mL and 122,6 μg/mL, respectively. These results were compared with a study on normal Embryonic Fibroblasts (REF), which confirmed the low toxicity of MH in normal cells (IC50 = 486,9 μg/mL). An MH concentration of 62.5 μg/mL slightly inhibited hexokinase activity in a normal cell, while it significantly inhibited hexokinase activity in breast cancer cells. Furthermore, MH induced a reduction in ATP concentration, pyruvate, and acidity levels in cancer cells, which is associated with hexokinase inhibition [59]. According to a study by (Malaisse, et al. [60]), D mannoheptulose hexaacetate increased biological efficiency by effectively crossing the cell membrane with subsequent intracellular hydrolysis. The ester itself did not affect D-glucose phosphorylation but allowed inhibition of glycolysis by the unesterified sugar [60]. This fact may be useful for designing new glucose hexokinase inhibitors and improving their pharmacokinetic properties.
Glucosamine Derivatives: Derivatives of glucose in which the hydroxyl group in position 2 has been replaced by an amino group, glucosamine derivatives, have been reported as anticancer agents with potential HK2 inhibitory activities (Figure 6) [61]. According to a High-Throughput Study (HTS hits), glucosamine derivatives were identified as potential selective hexokinase 2 inhibitors (HK2 IC50 = 6,3 μM; HK1 IC50 = 2,0 μM) (1) [62]. Based on SAR studies, optimization of their structure leads to the discovery of new and more selective HK2 inhibitors (Figure 7). The most promising glucosamine derivative 4’-chloro-N-((2S,3R,4R,5S,6S)6((2,3,dichlorophenylsulfonyl) methyl)2,4,5-trihydroxytetrahydro-2H-pyran-3-yl)biphenyl-3-sulfonamide (2) showed more than 100-fold higher selectivity against HK2 compared to HK1 with an average value of IC50 = 0,0079 μM and 1,0 μM, for HK2 and HK1, respectively. The inhibitory activity of glucosamine derivatives was evaluated in the UM-UC-3 cancer cells line, which had over-expressed HK2 and minimal HK1 expression [62]. Crystallographic studies confirmed the flexibility of the HK2 binding site to adopt an “induced-fit” conformation with inhibitors. (Lin, et al. [62]) demonstrated a dual mechanism of inhibition of this novel inhibitor, which binds to both the G6P binding pocket and the glucose- binding pocket.
Benserazide (BENZ): BENZ is a well-known drug for the treatment of Parkinson’s disease. BENZ is a decarboxylase inhibitor approved by the FDA but has been recently identified as a hexokinase 2 inhibitor. In silico studies have shown that BENZ occupies the active binding pocket in HK2, and the pyrogallol part of the BENZ structure interacts with the substrate (glucose) binding site. BENZ shows reasonable selectivity because it prefers one isoform of this enzyme, such as HK2 [63]. The inhibition measurement data demonstrated that the IC50 values for the HK2, HK1, and HK4 isoenzymes are 5.52 ± 0.17 μM, 25.13 ± 0.24 μM and 40.53 ± 2.94 μM, respectively. Their MST binding assay demonstrated that BENZ shows a high affinity for HK2 with a value of equilibrium dissociation constant (Kd) of 149 ± 4.95 μM. A comparison of the MTS results for BENZ with three reported HK2 inhibitors (3-BrPA, 2-DG, Met) indicated that BENZ was a compound with the highest affinity for glucose (Table 2). (Li, et al. [63]) postulate competitive and non-competitive inhibition of BENZ to HK2 by binding BENZ to the active site of the enzyme by changing the HK2 conformation. The kinetic enzyme assay showed that the Km value of glucose increased in a dose-dependent manner, whereas the Vmax value of glucose decreased after BENZ treatment. According to the data from Li, BENZ has shown cytotoxicity to various tumour cells such as SW480, Lovo, HCT116, MCF-7, and SMMC-7721, showing cytotoxic effects only in cancer cells, particularly SW480 (IC50 = 143 ± 7.0 μM). Furthermore, an in vivo test using a mouse xenograft model showed strong cell growth inhibitory activity under BENZ treatment. Experiments have confirmed that HK2 was a target of BENZ and this drug affected glucose metabolism [63]. But recent studies [64] have shown that BENZ reduced tumour growth and progression through a different mechanism than targeting HK2. In these studies, BENZ is DNA repair activating agent and it may prevent or retain the progression of breast tumours and their metastases.
Benitrobenrazide BNBZ: Recently, a new compound benitrobenrazide ((E)-4-Nitro-N′-(2,3,4-trihydroxybenzylidene, BNBZ) has been reported as a promising HK2 inhibitor. BNBZ was identified by structure-based virtual ligand screening as a strong HK inhibitor. In vitro and in vivo studies confirmed the inhibitory effect on HK2 and promising antitumour activity. BNBZ displayed inhibition of HK2, HK1, and HK4 activity with IC50 values of 0.53 ± 0.13 μM, 2.20 ± 0.12 μM, 4.17 ± 0.16 μM, respectively. The enzyme kinetic measurements indicated competitive inhibition of the glucose substrate by BNBZ with high affinity for the substrate with a Km value of 4.99 ± 0.41μM [65,66]. In its structure, BNBZ has an aromatic hydrophobic substituent and a highly polar fragment, which allows greater affinity for the active site and more effective pharmacokinetic properties (Figure 8). The prediction of molecular coupling demonstrated that BNBZ exhibits competitive inhibition occupying the same binding pocket as glucose [65]. According to the structure-activity research, the trihydroxybenzene fragment in BNBZ is required for its HK2 inhibitory activity [67]. The affinity of BNBZ dihydroxybenzylidene derivatives with other locations of hydroxyl groups toward the inhibitory effect on HK2 was investigated in vitro using HepG2 and HUH7 cell lines as a biological model.
A comparison of the results with those of the parent BNBZ indicates that only BNBZ exhibited satisfactory inhibitory activity in vitro and within the cell. None of the BNBZ derivatives showed inhibition of the enzyme in vitro, but some showed HK2 inhibitory activity in cell lines. The reason for this may be the tendency of derivatives to form aggregates. MST studies demonstrated that only one of the BNBZ derivatives (2,4-dihydroxy derivative) showed moderate affinity for HK2 (Km=58.9 μM), while other BNBZ derivatives did not show binding affinity to HK2 [67]. According to cell viability tests of four pancreatic cancer cell lines expressing HK2, BNBZ has the strongest effect on cancer cells with high overexpression of HK, such as SW1990 and MIApaca-2 cells with an IC50 value of approximately 25 μM. Moreover, BNBZ has shown no obvious toxicity to normal cells LO2, L929, and Vero with an IC50 value above 400 μM [66]. Studies by (Zheng, et al. [66]) have shown that the anticancer effects of BNBZ involve inhibition of HK2 activity as a factor that causes a decrease in glucose uptake and lactate production and an increase in the production of Reactive Oxygen Species (ROS), resulting in inhibition of cancer cell proliferation and inducing apoptosis.
Steroid from Ganoderma: Ganoderma sinense are important wood-decaying fungi occurring throughout the world. Their strains are a source of steroids which are a natural inhibitor of hexokinase. Ganoderma species are characterized by a wide biological activity and have been used for thousands of years in folk Chinese medicine [68]. One of the main active components from Ganoderma was sterols selected as inhibitors of HK2. According to the structure-based virtual ligand screening, the highest binding affinity to HK2 was shown by one of the thirteen sterols from Ganoderma such as (22E,24R)-6-β- methoxyergosta7,9(11),22-triene-3β,5α-diol. The MST assay showed the equilibrium dissociation constant Kd equal to 114.5 ± 2.7 μM, which means a strong binding affinity. This steroid exhibited strong enzyme inhibitory effects against HK2 with IC50 values of 2.06 ± 0.15 μM, with non-competitive inhibition [68]. On the other hand, no inhibitory effect on other isoforms of HK was shown in these studies [68], which hinders conclusions on the selectivity of the compound. According to the cytotoxicity test, this steroid exhibited 4-fold selectivity against cancer cells SW1990 (IC50 = 5.05 ± 0.17 μM) versus normal cells Vero (IC50 = 22.59 ± 1.24 μM) and strongly inhibited cancer cell growth compared to known inhibitors: Benserazide and Metformin [63].
Germicidins: Other natural compounds such as new α-pyrone derivatives and their known analogues, named germicidins were screened for HK2 inhibition activity in vitro. These compounds were discovered from Streptomyces sp. 18A01 isolated from a sponge specimen collected from the sea area in Yongxing island of the South China Sea. One of these germicides exhibited significant inhibitory effects against HK2 with an IC50 value of 5.13 ± 0.53 μM. It was approximately the same result compared to the IC50 value of the known benserazide inhibitor used in the positive control (IC50 = 5.52 μM) [69]. Benzimidazoles: Benzimidazoles exhibit a wide spectrum of biological activities, including antihypertensive, anti-inflammatory, anti-ulcer, anthelmintic, antimalarial, antibacterial, antioxidant, and anticancer [70]. Benzimidazoles such as Fenbendazole, Albendazole, and Mebendazole are popular drugs used in the treatment of parasites in both human and veterinary medicine [70-72]. All three compounds have shown potent selective inhibitory effects on the growth of various cancer cells through modulation of multiple cellular pathways and depolymerizing microtubules. [71-75]. Benzimidazoles have been observed to reduce the expression of enzymes involved in glycolysis, including HK2 [71-75]. Molecular Docking Analysis demonstrates that Fenbendazole (FZ), Albendazole, and Mebendazole have binding interactions with HK2. In vitro analysis results showed inhibition of hexokinase activity by Fenbendazole, Albendazole, and Mebendazole with IC50 values of 0.25 ± 0.1 μM, 2.5 ± 0.8 μM and 10.0 ± 1.2 μM, respectively. The most promising benzimidazole as a potential HK2 inhibitor is an FZ, drug used against parasites in the digestive tract in animals [73]. Albendazole and mebendazole are drugs used for the treatment of parasitic diseases in humans. Many benzimidazole derivatives were evaluated in clinical trials for different anticancer cures.
Ongoing clinical trials do not have enough data to assess whether benzimidazole antibiotics can be used to treat cancer in humans [74]. Fenbendazole was applied in the in vitro and in vivo treatment of human Non-Small Cell Lung Carcinoma (NSCLC) [75]. FZ causes reduced glucose uptake in H460 and A549 cell lines after treatment with a drug concentration of 1 μM. The same concentration of FZ results in a significant reduction of HK2 expression. In silico studies suggested that FZ can bind to the glucose pocket in HK2 and thus mimic a substrate or a product of HK2. Inhibition of the activity of the HK2 assay in H460 cells and lysates of H460 and A549 cell lysates at a dose of 1 μM confirms the inhibitory activity of FZ. Fenbendazole inhibits purified HK2 in a dose-dependent manner (5-10 μM) [75]. This is in contradiction to the (Jang, et al. [76]), report in which FZ and other benzimidazole, such as oxibendazole, have been shown to have no effect on HK2 activity (in vitro) at a concentration of 1 and 10 μM. Poor pharmacokinetic parameters of benzimidazoles [76] and lack of a fully understood mechanism of action prompt further research on the potential of benzimidazoles as anticancer drugs.
Compounds that Disrupt the VDAC1-HK2 Complex
(Figure 9) The interaction of HK2 with VDAC1 is important for the regulation of glucose metabolism and the apoptosis process in cancer cells. Disrupting the HK2–VDAC1 binding complex by small molecules could inhibit the growth and survival of cancer cells. In this review, compounds with HK2-VDAC1 activity are reported to inhibit the activity in cell lines by dissociating HK2 from VDAC1 (Figure 10). Inhibitors such as LD and Met have been shown to directly affect HK, as well as disrupt the HK2-VDAC1 complex. When designing new drugs based on HK2-VDAC1 interference, attention should be paid to their cardiotoxic effects; it was reported that disruption of HK2 from mitochondria causes detrimental effects on myocardial function [77].
Bromopyruvate (3-BrPa): Investigation of the influence of key glycolytic enzymes on hepatocellular carcinomas led to the discovery of 3-BrPa as an inhibitor of the glycolysis pathway [78]. This compound has shown promising anticancer activity both in vivo and in vitro [78-83]. Studies show that 3-BrPa induces covalent modification of the enzyme dissociates HK2 from VDAC and releases the apoptosis- inducing factor. Incubation of HL-60 cells with 100.0 μM of 3-BrPa results in a significant dissociation of HK2 from mitochondria [80]. In cells treated with 3-BrPa, a decrease in HK2 expression is also a decrease in HIF-1α expression [81]. 3-BrPa is known as an alkylating agent and it can react with the cysteine residue in the protein chain, thus inhibiting hexokinase enzymatic activity [80]. Moreover, 3-BrPA has shown dose-dependent toxicity to the liver and duodenum [82]. (Hwan Jun Jae, et al. [83]) conducted studies on the evaluation of the anticancer effect and hepatotoxicity in a rabbit VX2 hepatoma model administrating 3 BrPA intravenously in small-doses 3-BrPA (25 mL at a 1 mM concentration, n = 10) and high-dose (25 mL in at 5 mM concentration, n = 10) and doxorubicin as a positive control (1.6 mg doxorubicin/0.4 mL lipiodol, n = 10). The tumour necrosis rate values were 93% ± 7.6 (high dose 3-BrPa), 62% ± 20.0 (low dose 3-BrPa), and 99% ± 2.7 (lipiodol-doxorubicin), respectively. The hepatotoxicity observed in the 3-BrPA group was comparable to that in the lipiodol- doxorubicin group. These results indicated limited efficacy of 3-BrPa application compared to conventional methods.
Methyl Jasmonate (MJ): Methyl Jasmonate (MJ), the plant stress hormone, is a natural cyclopentanone lipid. MJ exhibited anticancer activity through multiple mechanisms [84]. The main targets of MJ treatment in cancer cells are HK2-VDAC complex, Reactive Oxygen Species (ROS), 5 lipoxygenase (5-LOX) pathway, MAPK signaling pathway and NF-κB pathway. The anticancer effects of MJ have been assessed in many types of various human and mouse cancer cell lines such as melanoma, lymphoma cells, prostate, melanoma, lymphoblastic leukemia, and breast [85]. All cytotoxic effects of MJ on cells were observed at millimolar concentrations [84]. It is worth noticing that MJ induces ATP depletion in cancer cells but not in normal cells [86]. A number of MJ toxicity studies have shown that the compound is non-toxic to normal cells. The U.S. Federal Environmental Protection Agency has confirmed that MJ is non-toxic to humans and is a substance commonly consumed in fruits and is safe for humans. In addition, MJ as a food additive has been approved by the Food and Agriculture Organisation/World Health Organisation (FAO/WHO) [84]. According to (Goldin, et al. [27]), the proposed mechanism of MJ inhibitory effect on the hexokinase activity involves MJ binding to HK, which interferes with the HK-VDAC complex, hence enabling dissociation of HK from VDAC. Hexokinase released from the HK-VDAC complex is inhibited by G6P and has a lower affinity for ATP. MJ influences two other hexokinase isoforms: mitochondria-associated hexokinases HK1 and HK2. Destruction of the HK2-VDAC1 complex by MJ resulted in loss of mitochondrial function, among others, by inducing the transition of mitochondrial permeability and subsequent release of cytochrome c from mitochondria, leading to cell necrosis and apoptosis [86,87]. The discovery of MJ as a candidate for cancer treatment has resulted in the design and synthesis of derivatives of MJ [85]. In vitro studies of therapeutic effects of new MJ derivatives on glioblastoma brain tumours that are aggressive and malignant and highly lethal are due to overexpression of HK2. One of the novel analogs of MJ (Figure 11) more effectively reduces glioblastoma cell viability than MJ [85]. This 1,2,4-oxadiazole derivative inhibited HK2 more strongly and irreversibly than MJ with a value IC50 of 0.27 μM, and 7.47 μM, respectively [88]. The mechanism of action involved dissociation of the HK2-VDAC complex associated with mitochondria [85].
Clotrimazole (CTZ) and Bifnazole: Clotrimazole-an azole derivative is a popular antifungal drug. Investigations suggest that it is a promising agent for other disease treatments such as sickle cell disease and malaria. A number of reports have suggested that clotrimazole may be a drug for cancer treatment [89]. The mechanism of action of CTZ in cancer cells reported by (Pastorino, et al. [90]) involves the dissociation of HK2 from VDAC. Dissociation of HK2-VDAC complex increases pro-apoptotic BAX binding to the mitochondrion, mitochondrial membrane porosity associated with the release of cytochrome c, and subsequent cell death. This mechanism of action of CTZ has been confirmed by other groups [30,91]. Another antifungal agent, such as bifonazole, affects hexokinase activity by disconnecting it from the mitochondria of B16 melanoma cells. The IC50 in the detachment of HK2 from mitochondria by bifonazole and clotrimazole was equal to 10–15 μM [91]. Recent research by Cristiane M. Furtado and co-workers [92] suggested that clotrimazole affects the activity of glucose 6-phosphate dehydrogenase and glycolytic enzymes such as hexokinase, pyruvate kinase and phosphofructokinase in breast cells and dysregulates glycolysis, which can reduce of tumour progression. Treatment of metastatic cell lines MCF 7, MDA-MB-231, and normal breast cells MCF10A with different concentrations of clotrimazole (0– 100μM) under 24 h incubation time showed inhibition of cancer cell growth, especially in the more aggressive lines such as MDA-MB-231. CTZ has been established to inhibit the activity of HK2 in a dose-dependent manner. Moreover, CTZ showed a more pronounced inhibitory effect on HK2 activity in cancer cell lines in comparison with normal cells [92]. The 100 μM CTZ dose inhibited the activity in normal cells (MCF10A cells), in 46% HK, while the HK activity in MCF-7 and MDA-MB-231 cells was inhibited in 90% and 96%, respectively, by the same concentration of CTZ. Alternatively, (Carpi, et al. [93]) proposed the mechanism of action of CTZ through direct inhibition of enzymes [93]. Their investigation suggests that clotrimazole causes cell-cycle arrest and apoptosis in human melanoma cells in correlation with inhibition of HK2. Their study shows that clotrimazole induced cell death in A375 melanoma cells and caused a reduction in HK expression and activity under treatment with 10 μM of CTZ. Moreover, their report suggested the selectivity of clotrimazole against cancer cells because this drug significantly inhibited the activity of the HK and induced apoptosis in cancer cells without major changes in proliferating human keratinocytes. This effect depends on the higher rate of glycolysis in melanoma cells in comparison to normal cells. The high activity of CTZ against multiple targets makes its selectivity low. Clinical studies have shown that oral use of CTZ causes cytochrome P450 enzyme inhibition-thereby limiting its therapeutic value [94].
Flavonoids: Flavonoids are natural polyphenolic compounds that exhibit anticancer activity by inhibiting glycolysis. According to the anticancer effect of oroxylin A on non-small cell lung carcinoma, this flavonoid influences the c-Src / AKT / HK II pathway and interferes with HK2 binding to VDAC. [95] Synthetic modification of another flavonoid, genistein, resulted in novel polyphenolic derivatives: (5-hydroxy- 7-(2-hydroxy-3-(piperidin-1-yl)propoxy)-3-(4-(2-hydroxy-3- (piperidin-1-yl)propoxy)phenyl)-4H chromen-4-one), gen-27 (Figure 12) [96]. In vitro and in vivo studies have demonstrated promising anticancer effects of Gen-27 on human breast cancer. Inhibition of breast cancer cell growth by Gen-27 is concentration- and time-dependent (10-30 μM) and shows stronger effects than its parent compound, genistein. The mechanism of action of Gen-27 is to reduce the expression of mitochondrial HK2. It was confirmed that gen-27 dissociated HK2 from VDAC1 and changed the translocation of HK2 from the mitochondria to the cytosol [96].
Another Class of Potential Anticancer Approaches: Reducing Expression HK2
Trastuzumab, an approved anticancer drug, is a monoclonal antibody that targets the HER2 receptor. HER2 overexpression occurs in some types of breast cancer with a poor prognosis. The binding of trastuzumab to HER2 receptors affects the Akt/PI3K and MAPK pathways, which influence glycolysis. Treatment with antiHER2 compounds in xenografts derived from MDA-MB-453 breast tumour cells resulted in a significant decrease in the expression of HK2 and GLUT1, which may be related to the inhibition of tumour growth [97]. In vitro studies on the effects of Trastuzumab on breast cancer cells showed that the drug causes a decrease in HK activity [98]. In a novel approach to cancer treatment, the usage of siRNA (Small Interfering RNA) to silence specific gene expression is considered [99]. Under treatment, anaplastic thyroid cancer cells with lentiviral Small Hairpin RNA (shRNA) reduction of HK2 expression and inhibition of cell proliferation were observed. Lenti-HK2 shRNA treatment is a method of prolonged repression of the expression of a targeted HK2 gene [100]. shRNA gene knockdown is characterized by high specificity because it interferes with the expression of one HK isoform [101]. Small hairpin RNA can stay in cells much longer than siRNA, so shRNA exhibits long-term effects. The vector expressing shRNA against HK2 decreased glucose uptake and total HK activity. Combination therapy using lenti-HK2 shRNA and 131I Human Sodium Iodide Symporter (HNIS) to treat anaplastic thyroid cancer cells in an animal model showed a significantly stronger antitumour effect than single therapy [100]. Another study showed that down-regulation of HK2 gene expression by shRNA inhibited LoVo colon cancer cell proliferation in vitro and tumour growth in vivo [101].
Chemotherapy and radiation therapy are most used for cancer treatment. The major challenge in the treatment of cancer is the chemo- and radiation-resistance of cancer cells, in factors related to disease progression and therapy failure. It is assumed that HK2 overexpression is associated with chemo- and radiation-resistant phenotypes [102,103]. HK2 inhibitors as potential adjuvant agents have been shown to restore the sensitivity of cancer cells to drugs available on the market. The selected studies presented in Table 3 confirm that HK2 inhibitors synergistically enhance the therapeutic effect of known anticancer drugs [102-121].
Table 3: Synergistic effect in cancer treatment in combinatorial therapy with HK2 inhibitors and commercially available anticancer drugs.
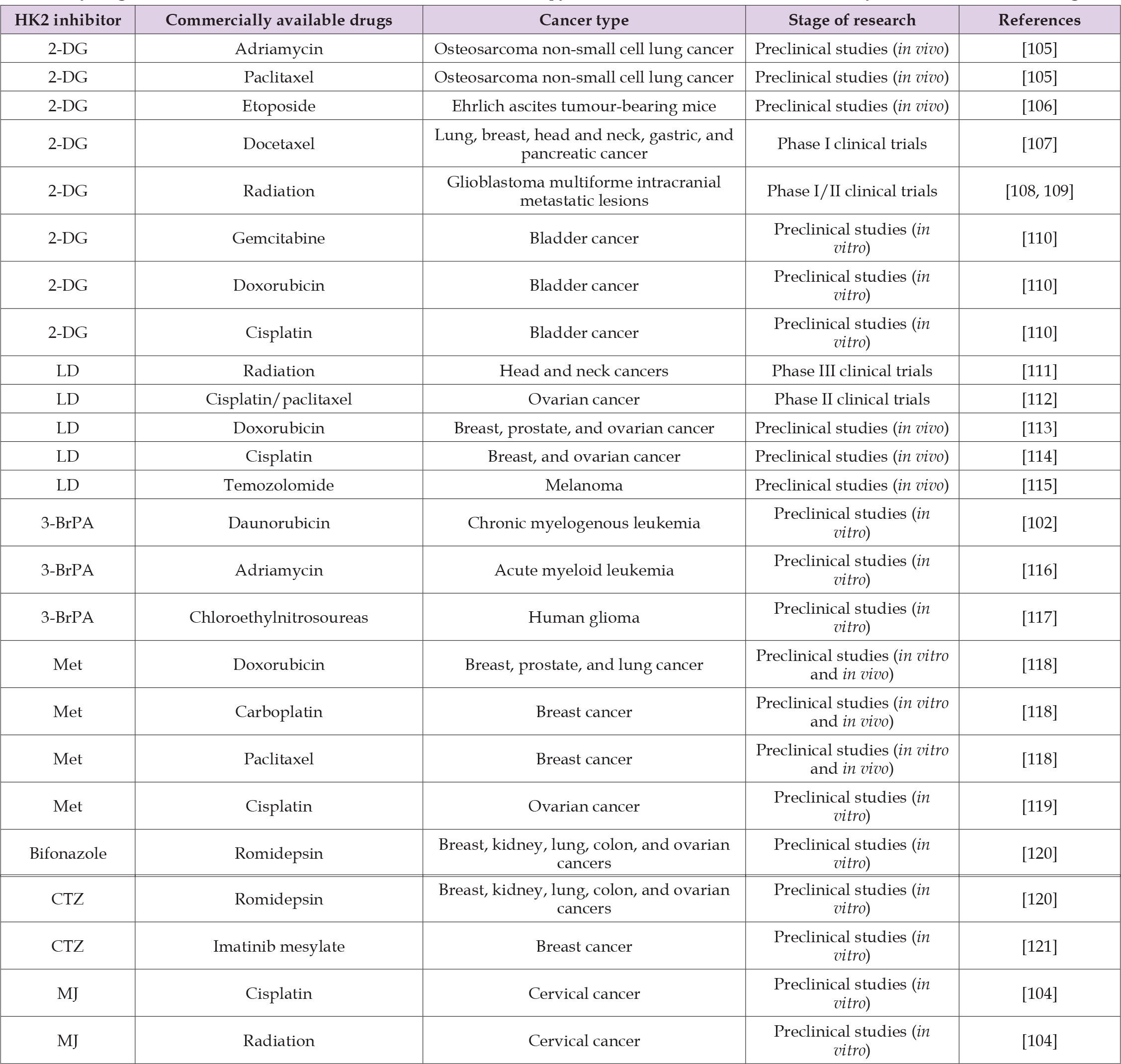
Chemoresistance
Drug resistance is often associated with higher energy requirements and increased glycolysis rates. By comparing human breast cancer cells MCF-7 and a drug-resistant cell line derived from them, data proved that the drug-resistant cell line showed a 3-fold higher rate of glycolysis and, consequently, a higher demand for ATP [103]. The complete depletion of ATP in cancer cells mediated by HK2 inhibition may be one of the main reasons for overcoming drug resistance in cancer. In addition, the reduction of ATP levels by HK2 inhibitors has shown a synergistic effect in combination therapy on growth inhibition and prevention of recurrence in several types of cancer cells [106,108,109,110]. In solid tumours, the expression of specific proteins like HIF-1α, GLUT1, HK2, and LDH significantly increased [106,108]. Cell hypoxia is considered a key factor in drug resistance. Therefore, combining HK2 inhibitors with typical anticancer agents may be a way to simultaneously target hypoxic and aerobic cells in tumours [110].
(Valera, et al. [110]) assumed that the synergistic effect of combination therapy with docetaxel and 2-DG is a result of effective targeting of rapidly growing tumours in normoxia by docetaxel, while 2-DG targets tumour cells in hypoxia. Cells treated with drugs such as cisplatin, which cause DNA damage, can become resistant to treatment by switching on DNA repair mechanisms. HK2 inhibitors usage and subsequent glycolysis inhibition retained repair mechanisms in cancer cells, and damages cannot be easily removed because their ATP level is reduced [105]. Lonidamine has been reported to interfere with the repair process of damaged DNA during combinatory therapy with cisplatin [114].
Radioresistance
Radioresistance can result from a variety of mechanisms. Low oxygen levels in solid tumours are one of the factors responsible for radioresistance. It is believed that a hypoxia-induced decreasing in the amount of reactive oxygen species, and consequently a reduction of DNA damage, is due to radiation therapy. Further defense mechanisms against radiation include detoxification of Reactive Oxygen Species (ROS) and repair of DNA damage after ionizing irradiation. Glucose metabolism plays a significant role in these processes because energy is required to activate repair mechanisms [122]. The use of HK2 inhibitors can prevent or reverse these effects. One of the HK2 inhibitors, 2 DG, has been used in clinical trials I/II phase as an adjunct to radiotherapy. The results showed an increased survival while improving the quality of life and selective sensitivity to radiation [108,109]. In phase III of clinical trials, LD usage improved the efficacy of radiotherapy. Potentially Lethal Damage (PLD) repair has been reported to be an energy-dependent process of repairing DNA damage after radiation therapy. It has been assumed that LD disturbs this process, and enhances the effect of radiation without toxic effects to normal tissues [111].
Sensitization
The main problem with the use of high doses of radiation and anticancer drugs is the wide range of side effects during and after treatment. According to some reports, it has been proven that the use of HK2 inhibitors during anticancer therapy with known pharmaceuticals or radiation allows the use of lower doses of these agents which are effective in inhibiting growth [111,115,116]. Preclinical studies by (Iliopoulos et al. [118]) on mouse xenografts have revealed that combined treatment with metformin and doxorubicin allows reduced dosage of doxorubicin. Treatment with an oral dose of 200 μg/mL metformin with 1g/kg doxorubicin injection caused complete tumour regression and no detectable recurrence for at least 65 days. Comparable effects were observed under 4g/kg injection treatment, which is associated with a 4-fold reduced dose of doxorubicin in cancer treatment.
Physiological features of cancer cells, such as uncontrolled growth, weak specialization of organelles, and different metabolism of glucose under aerobic conditions, distinguish them from normal cells. HK2 is the first enzyme involved in the glycolysis pathway, converting glucose to glucose-6-phosphate and its overexpression in cancer cells, and this has been confirmed in many studies. In addition to glucose phosphorylation, the function of mitochondrial HK2, which is associated with VDAC, is to protect cancer cells from apoptosis. Therefore, HK2 is a potent target in cancer treatment. This review presents the actual knowledge about hexokinase 2 inhibitors as potential agents in cancer therapies. These compounds have shown anticancer activity both in vitro and in vivo. The promising anticancer effects of HK2 inhibitors have been discussed in many studies. Only one HK2 inhibitor has been approved for clinical use in humans, namely, Lonidamine (LD); so much more research is needed to determine safe, highly specific, and efficient inhibitors. An important aspect in the design of HK2 inhibitors is selectivity toward other isoforms, which is related to the safety of these drugs. HK2 inhibitors should not affect HK1, HK3, and HK4 isozymes, which are needed to maintain normal cell functions High-specificity inhibitors for one HK2 isoform have been found to reduce the side effects of cancer therapy. Conducting studies on distinct functions, structural characteristics, and expression of HK2 from other isoforms can be used for preferential inhibition of HK2 over other isoforms in cancer. The review suggests that modification of already known inhibitors may be the way to find novel, selective, and effective inhibitors with good pharmacokinetic properties. The HK2 inhibitors discovered are characterized by selective activity against many cancers with insignificant effects on normal tissues. However, its use as a single anticancer agent is not effective enough. HK2 inhibitors appear to be promising as an adjuvant in combined therapies. The effects of HK2 inhibitors on drug-sensitive and drug-resistant cancer cells are a key area of research. Novel approaches awaiting further development could help us evolve novel research strategies and anticancer therapies.
The authors declare that there is no conflict of interest regarding the publication of this paper.
The research was financially supported by BKM-638/RCH- 2/2022.


